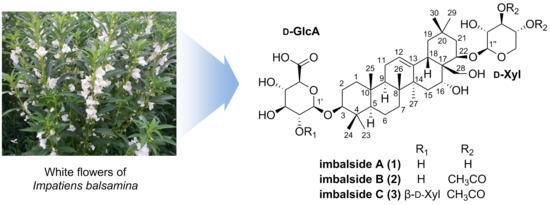
Three New Oleanane-Type Triterpenoidal Glycosides from Impatiens balsamina and Their Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Cytotoxicity Assessment
2.3. Anti-Neuroinflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Imbaloside A (1)
3.3.2. Imbaloside B (2)
3.3.3. Imbaloside C (3)
3.4. Acid Hydrolysis of 1–3 and Sugar Analysis
3.5. Cytotoxicity Assessment
3.6. Assessment of NO Generation and Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imam, M.Z.; Nahar, N.; Akter, S.; Rana, M.S. Antinociceptive activity of methanol extract of flowers of Impatiens balsamina. J. Ethnopharmacol. 2012, 142, 804–810. [Google Scholar] [CrossRef]
- Autore, G.; De Tommasi, N.; Aquino, R.; Garofalo, L.; Borrelli, F.; Tartaglia, D.; Mascolo, N. A new acetophenone derivative and inhibitory action of Muehlenbeckia volcanica on gastrointestinal transit in mice. Phytother. Res. 1996, 10, 202–206. [Google Scholar]
- Ding, Z.-S.; Jiang, F.-S.; Chen, N.-P.; Lv, G.-Y.; Zhu, C.-G. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. Molecules 2008, 13, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Oku, H.; Ishiguro, K. Cyclooxygenase-2 inhibitory 1, 4-naphthoquinones from Impatiens balsamina L. Biol. Pharm. Bull. 2002, 25, 658–660. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guo, Z.; Wang, K.; Zhang, X.; Lou, Y.; Zhao, Y.-Q. Two new 1, 4-naphthoquinone derivatives from Impatiens balsamina L. flowers. Phytochem. Lett. 2015, 14, 8–11. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kwon, O.W.; Park, H.B.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Wasabisides A–E, lignan glycosides from the roots of Wasabia japonica. J. Nat. Prod. 2016, 79, 2652–2657. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Diterpenes from the trunk of Abies holophylla and their potential neuroprotective and anti-inflammatory activities. J. Nat. Prod. 2016, 79, 387–394. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Lignan glycosides from the twigs of Chaenomeles sinensis and their biological activities. J. Nat. Prod. 2015, 78, 1174–1178. [Google Scholar] [CrossRef]
- Lee, T.H.; Khan, Z.; Kim, S.Y.; Lee, K.R. Thiohydantoin and hydantoin derivatives from the roots of Armoracia rusticana and their neurotrophic and anti-neuroinflammatory activities. J. Nat. Prod. 2019, 82, 3020–3024. [Google Scholar] [CrossRef]
- Lee, T.H.; Khan, Z.; Subedi, L.; Kim, S.Y.; Lee, K.R. New bis-thioglycosyl-1, 1′-disulfides from Nasturtium officinale R. Br. and their anti-neuroinflammatory effect. Bioorgan. Chem. 2019, 86, 501–506. [Google Scholar] [CrossRef]
- Suh, W.S.; Kwon, O.K.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Secoiridoid glycosides from the twigs of Ligustrum obtusifolium possess anti-inflammatory and neuroprotective effects. Chem. Pharm. Bull. 2018, 66, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Choi, S.Z.; Son, M.W.; Kim, K.H.; Lee, K.R. Two new phenolic compounds from the white flower of Impatiens balsamina. Phytochem. Lett. 2015, 14, 215–220. [Google Scholar] [CrossRef]
- Kim, C.S.; Bae, M.; Oh, J.; Subedi, L.; Suh, W.S.; Choi, S.Z.; Son, M.W.; Kim, S.Y.; Choi, S.U.; Oh, D.C.; et al. Anti-neurodegenerative biflavonoid glycosides from Impatiens balsamina. J. Nat. Prod. 2017, 80, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Subedi, L.; Oh, J.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Bioactive triterpenoids from the twigs of Chaenomeles sinensis. J. Nat. Prod. 2017, 80, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, J.H.; Moon, E.; Kim, S.Y.; Lee, K.R. New triterpenoids from the tubers of Corydalis ternata: Structural elucidation and bioactivity evaluation. Planta Med. 2011, 77, 1555–1558. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.K.; Choi, S.U.; Lee, K.R. Triterpene saponins from Pleurospermum kamtschaticum and their biological activity. Chem. Pharm. Bull. 2012, 60, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, M.R.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Pouységu, L.; Quideau, S.; Rojas, L.B.; Porcar, C.R.; Lacaille-Dubois, M.-A. Oleanane-type glycosides from Pittosporum tenuifolium “variegatum” and P. tenuifolium “gold star”. Phytochemistry 2017, 140, 166–173. [Google Scholar] [CrossRef]
- Mroczek, A.; Kapusta, I.; Janda, B.; Janiszowska, W. Triterpene saponin content in the roots of red beet (Beta vulgaris L.) cultivars. J. Agric. Food Chem. 2012, 60, 12397–12402. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Espineli, D.L.; Shen, C.-C. New triterpenes from Barringtonia asiatica. Chem. Pharm. Bull. 2011, 59, 778–782. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Cytotoxic triterpenoids from Berberis koreana. Planta Med. 2012, 78, 86–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, K.W.; Cha, J.M.; Choi, S.U.; Lee, K.R. A new triterpene glycoside from the stems of Lagerstroemia indica. Arch. Pharm. Res. 2016, 39, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.S.; Park, K.J.; Kim, D.H.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. A biphenyl derivative from the twigs of Chaenomeles speciosa. Bioorgan. Chem. 2017, 72, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Bioactive triterpenoids from twigs of Betula schmidtii. Bioorgan. Chem. 2018, 77, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, M.; Lim, S.; Sung, S.; Kim, Y. Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1β by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br. J. Pharmacol. 2009, 156, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Shoji, N.; Umeyama, A.; Taira, Z.; Takemoto, T.; Nomoto, K.; Nizukawa, K.; Ohizumi, Y. Chemical structure of hosenkol-A, the first example of the natural baccharane triterpenoid of the missing intermediate to shionane and lupane. J. Chem. Soc. Chem. Commun. 1983, 871–873. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Saitou, N.; Yoshikawa, K.; Kan, Y.; Arihara, S. Hosenkosides A, B, C, D and E, novel baccharane glycosides from the seeds of Impatiens balsamina. Tetrahedron 1994, 50, 4973–4986. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Saitou, N.; Yoshikawa, K.; Nagai, M.; Arihara, S. Hosenkosides F, G, H, I, J and K, novel baccharane glycosides from the seeds of Impatiens balsamina. Chem. Pharm. Bull. 1994, 42, 1422–1426. [Google Scholar] [CrossRef] [Green Version]
- Shoji, N.; Umeyama, A.; Yoshikawa, K.; Nagai, M.; Arihara, S. Baccharane glycosides from seeds of Impatiens balsamina. Phytochemistry 1994, 37, 1437–1441. [Google Scholar] [CrossRef]
- Lei, J.; Qian, S.; Jiang, J. A new ursane caffeoyl ester from the seeds of Impatiens balsamina L. J. China Pharmceutical Univ. 2010, 41, 118–119. [Google Scholar]



| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1ax | 40.2 | 1.65, overlap | 40.2 | 1.65, overlap | 40.3 | 1.64, overlap |
| 1eq | 1.01, overlap | 1.03, overlap | 1.02, overlap | |||
| 2ax | 27.1 | 1.72, overlap | 27.1 | 1.73, overlap | 27.2 | 1.73, overlap |
| 2eq | 1.97, overlap | 2.00, overlap | 2.02, overlap | |||
| 3 | 91.1 | 3.21, overlap | 91.1 | 3.22, overlap | 91.0 | 3.21, overlap |
| 4 | 40.4 | 40.4 | 40.5 | |||
| 5 | 57.3 | 0.82, d (11.6) | 57.3 | 0.82, d (11.6) | 57.3 | 0.80, d (11.5) |
| 6ax | 19.5 | 1.44, overlap | 19.5 | 1.46, overlap | 19.5 | 1.44, overlap |
| 6eq | 1.61, overlap | 1.61, overlap | 1.61, overlap | |||
| 7a | 34.4 | 1.64, overlap | 34.4 | 1.63, overlap | 34.4 | 1.63, overlap |
| 7b | 1.43, overlap | 1.42, overlap | 1.43, overlap | |||
| 8 | 41.0 | 41.0 | 41.0 | |||
| 9 | 48.3 | 1.66, overlap | 48.3 | 1.67, overlap | 48.3 | 1.66, overlap |
| 10 | 38.0 | 38.0 | 37.9 | |||
| 11 | 24.7 | 1.91, overlap | 24.7 | 1.92, overlap | 24.7 | 1.92, overlap |
| 12 | 124.2 | 5.30, brs | 124.2 | 5.30, brt (3.7) | 124.2 | 5.30, brt (3.7) |
| 13 | 144.6 | 144.6 | 144.6 | |||
| 14 | 42.7 | 42.7 | 42.7 | |||
| 15ax | 34.5 | 2.01, brd (13.5) | 34.6 | 2.02, overlap | 34.6 | 2.04, overlap |
| 15eq | 1.43, overlap | 1.43, overlap | 1.44, overlap | |||
| 16 | 70.4 | 4.25, brt (3.4) | 70.5 | 4.24, brt (3.5) | 70.5 | 4.23, brt (3.4) |
| 17 | 45.6 | 45.6 | 45.6 | |||
| 18 | 42.8 | 2.06, brd (13.0) | 42.8 | 2.10, d (13.2) | 42.8 | 2.10, d (13.1) |
| 19ax | 48.2 | 2.28, t (13.4) | 48.2 | 2.29, t (13.5) | 48.2 | 2.29, t (13.4) |
| 19eq | 1.00, overlap | 1.00, overlap | 1.01, overlap | |||
| 20 | 31.1 | 31.1 | 31.1 | |||
| 21ax | 40.2 | 1.74, dd (13.6, 3.4) | 40.4 | 1.78, dd (13.7, 3.9) | 40.4 | 1.78, dd (13.7, 3.9) |
| 21eq | 1.65, overlap | 1.69, overlap | 1.69, overlap | |||
| 22 | 78.8 | 4.28, dd (7.6, 3.9) | 78.8 | 4.28, dd (7.0, 3.9) | 79.8 | 4.28, dd (7.0, 3.9) |
| 23 | 28.7 | 1.09, s | 28.7 | 1.09, s | 28.4 | 1.09, s |
| 24 | 17.2 | 0.88, s | 17.2 | 0.89, s | 16.8 | 0.88, s |
| 25 | 16.4 | 1.00, s | 16.4 | 1.01, s | 16.4 | 1.01, s |
| 26 | 17.7 | 0.97, s | 17.7 | 0.98, s | 17.7 | 0.98, s |
| 27 | 27.3 | 1.40, s | 27.4 | 1.41, s | 27.4 | 1.40, s |
| 28a | 65.8 | 3.64, d (12.0) | 65.8 | 3.65, d (12.0) | 65.9 | 3.65, d (12.1) |
| 28b | 3.27, overlap | 3.29, d (12.0) | 3.29, d (12.0) | |||
| 29 | 32.6 | 0.93, s | 32.8 | 0.93, s | 32.8 | 0.93, s |
| 30 | 29.1 | 1.05, s | 28.9 | 1.05, s | 28.9 | 1.05, s |
| 1′ | 106.9 | 4.38, d (7.1) | 106.9 | 4.38, d (7.8) | 105.5 | 4.47, d (7.6) |
| 2′ | 75.6 | 3.28, overlap | 75.7 | 3.27, overlap | 83.4 | 3.51, overlap |
| 3′ | 78.1 | 3.41, overlap | 78.2 | 3.42, overlap | 78.3 | 3.61, overlap |
| 4′ | 73.8 | 3.50, overlap | 73.9 | 3.49, overlap | 73.8 | 3.51, overlap |
| 5′ | 76.8 | 3.68, overlap | 76.9 | 3.63, overlap | 77.1 | 3.61, overlap |
| 6′ | n/d | n/d | n/d | |||
| 1′′ | 102.8 | 4.38, d (7.1) | 103.1 | 4.49, d (7.5) | 103.1 | 4.49, d (7.6) |
| 2′′ | 74.5 | 3.23, overlap | 72.8 | 3.44, dd (9.1, 7.5) | 72.8 | 3.44, overlap |
| 3′′ | 77.0 | 3.41, overlap | 75.9 | 5.09, t (9.1) | 75.9 | 5.09, t (9.1) |
| 4′′ | 71.2 | 3.52, overlap | 70.8 | 4.87, td (9.6, 5.5) | 70.8 | 4.87, td (9.6, 5.4) |
| 5′′ax | 66.5 | 3.29, overlap | 63.6 | 3.46, dd (11.6, 10.0) | 63.6 | 3.46, overlap |
| 5′′eq | 3.95, dd (11.6, 4.9) | 4.06, dd (11.6, 5.5) | 4.06, dd (11.6, 5.4) | |||
| OAc-3′′ | 172.2 | 172.2 | ||||
| 21.0 | 2.08, s | 21.0 | 2.08, s | |||
| OAc -4′′ | 171.8 | 171.8 | ||||
| 20.7 | 2.02, s | 20.7 | 2.02, s | |||
| 1′′′ | 106.5 | 4.55, d (7.4) | ||||
| 2′′′ | 76.5 | 3.26, dd (8.9, 7.4) | ||||
| 3′′′ | 77.9 | 3.35, overlap | ||||
| 4′′′ | 71.4 | 3.49, overlap | ||||
| 5′′′ax | 67.3 | 3.17, overlap | ||||
| 5′′′eq | 3.84, dd (11.5, 5.3) | |||||
| Compound | IC50 (μM) 1 | |||
|---|---|---|---|---|
| A549 | SK-OV-3 | SK-MEL-2 | BT549 | |
| 1 | >30 | >30 | >30 | >30 |
| 2 | >30 | >30 | >30 | 26.4 |
| 3 | 29.8 | >30 | >30 | 29.2 |
| Cisplatin 2 | 0.9 | 2.0 | 1.1 | 1.2 |
| Compound | IC50 (μM) 1 | Cell viability (%) 2 |
|---|---|---|
| 1 | 41.0 | 109.2 ± 6.0 |
| 2 | 33.8 | 97.5 ± 2.1 |
| 3 | 34.8 | 83.7 ± 3.8 |
| L-NMMA 3 | 21.3 | 120.1 ± 11.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R.; Kim, C.S. Three New Oleanane-Type Triterpenoidal Glycosides from Impatiens balsamina and Their Biological Activity. Plants 2020, 9, 1083. https://doi.org/10.3390/plants9091083
Lee TH, Suh WS, Subedi L, Kim SY, Choi SU, Lee KR, Kim CS. Three New Oleanane-Type Triterpenoidal Glycosides from Impatiens balsamina and Their Biological Activity. Plants. 2020; 9(9):1083. https://doi.org/10.3390/plants9091083
Chicago/Turabian StyleLee, Tae Hyun, Won Se Suh, Lalita Subedi, Sun Yeou Kim, Sang Un Choi, Kang Ro Lee, and Chung Sub Kim. 2020. "Three New Oleanane-Type Triterpenoidal Glycosides from Impatiens balsamina and Their Biological Activity" Plants 9, no. 9: 1083. https://doi.org/10.3390/plants9091083