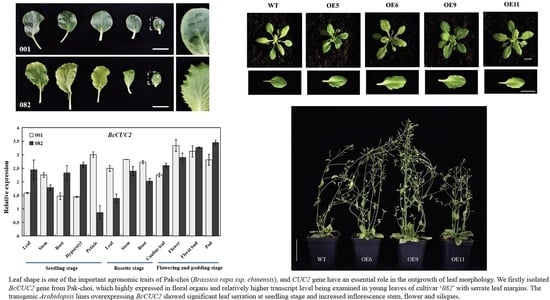
Ectopic Expression of BcCUC2 Involved in Sculpting the Leaf Margin Serration in Arabidopsis thaliana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. IAA and GA3 Treatments
2.3. Cloning and Sequence Analysis
2.4. Phylogenetic Tree Analysis
2.5. Subcellular Localization Assay
2.6. Ectopic Expression in Arabidopsis
2.7. Quantitative Real-Time PCR
3. Results
3.1. Cloning and Characteristic Analysis of BcCUC2
3.2. Phylogenetic Tree Analysis of BcCUC2
3.3. Expression Pattern Analysis of BcCUC2 in Pak-choi
3.4. Expression Analysis of BcCUC2 Gene under Hormone Treatment
3.5. Subcellular Localization Analysis of BcCUC2
3.6. Ectopic Expression of BcCUC2 Caused Leaf Margin Serration and Increased Lateral Branches in Arabidopsis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Primer | Sequence (5′-3′) |
|---|---|
| Cloning | |
| BcCUC2-F | ATGGACATTCCGTACTACCAC |
| BcCUC2-R | GTAATTCCATACGCAATCAAG |
| qRT-PCR | |
| qBcCUC2-F | CGGAGGCTCAGCAGAAGCAA |
| qBcCUC2-R | GGTGTAGCCGAGGGTTGTGG |
| qBcActin-F | GTTGCTATCCAGGCTGTTCT |
| qBcActin-R | AGCGTGAGGAAGAGCATAAC |
| qAtActin-F | TTGACAATTGATGCAAACAAT |
| qAtActin-R | CCATTGCTTAATTCCACGGAC |
| Overexpression | |
| KpnI-BcCUC2-F | GGGGTACCATGGACATTCCGTACTACCAC |
| BamHI-BcCUC2-R | CGGGATCCGTAATTCCATACGCAATCAAG |
| Gene Name | GenBank Accession | Plant Species | Protein Sequences |
|---|---|---|---|
| AtCUC1 | NM_112380 | Arabidopsis thaliana | MDVDVFNGWGRPRFEDESLMPPGFRFHPTDEELITYYLLKKVLDSNFSCAAISQVDLNKSEPWELPEKAKMGEKEWYFFTLRDRKYPTGLRTNRATEAGYWKATGKDREIKSSKTKSLLGMKKTLVFYKGRAPKGEKSCWVMHEYRLDGKFSYHYISSSAKDEWVLCKVCLKSGVVSRETNLISSSSSSAVTGEFSSAGSAIAPIINTFATEHVSCFSNNSAAHTDASFHTFLPAPPPSLPPRQPRHVGDGVAFGQFLDLGSSGQIDFDAAAAAFFPNLPSLPPTVLPPPPSFAMYGGGSPAVSVWPFTL |
| AtCUC2 | NM_124774 | Arabidopsis thaliana | MDIPYYHYDHGGDSQYLPPGFRFHPTDEELITHYLLRKVLDGCFSSRAIAEVDLNKCEPWQLPGRAKMGEKEWYFFSLRDRKYPTGLRTNRATEAGYWKATGKDREIFSSKTCALVGMKKTLVFYKGRAPKGEKSNWVMHEYRLEGKFSYHFISRSSKDEWVISRVFQKTTLASTGAVSEGGGGGGATVSVSSGTGPSKKTKVPSTISRNYQEQPSSPSSVSLPPLLDPTTTLGYTDSSCSYDSRSTNTTVTASAITEHVSCFSTVPTTTTALGLDVNSFSRLPPPLGFDFDPFPRFVSRNVSTQSNFRSFQENFNQFPYFGSSSASTMTSAVNLPSFQGGGGVSGMNYWLPATAEENESKVGVLHAGLDCIWNY |
| AtCUC3 | NM_106292 | Arabidopsis thaliana | MMLAVEDVLSELAGEERNERGLPPGFRFHPTDEELITFYLASKIFHGGLSGIHISEVDLNRCEPWELPEMAKMGEREWYFYSLRDRKYPTGLRTNRATTAGYWKATGKDKEVFSGGGGQLVGMKKTLVFYKGRAPRGLKTKWVMHEYRLENDHSHRHTCKEEWVICRVFNKTGDRKNVGLIHNQISYLHNHSLSTTHHHHHEALPLLIEPSNKTLTNFPSLLYDDPHQNYNNNNFLHGSSGHNIDELKALINPVVSQLNGIIFPSGNNNNDEDDFDFNLGVKTEQSSNGNEIDVRDYLENPLFQEASYGLLGFSSSPGPLHMLLDSPCPLGFQL |
| ChCUC1 | ACL14369.1 | Cardamine hirsuta | MDIVVFNGSERPRFEDDTLMPPGFRFHPTDEELITYYLLKKVLDSNFSCAAISQVNLNKSEPWELPEKAKMGEKEWYFFTLRDRKYPTGLRTNRATEAGYWKATGKDREIKSSKTKSLLGMKKTLVFYKGRAPKGEKSSWVMHEYRLDGKFSYHYISSSAKDEWVLCKVCLKSGVVNRETKSISSSTSAAGEFSSPGSTIAPIIDAFASEHVSCFSNDAAHANESFRTTYLPAPPPSLPPRQPRHIGDDVAFGQFMDFGFSGQIHYDAAAFFPNLPSLPPTALPPPPSFAMYGGGSTLSYWPFAL |
| ChCUC2 | ACL14370.1 | Cardamine hirsuta | MDIPYYHYDHGGDSQYLPPGFRFHPTDEELITHYLLRKVLDGCFSSRAIAEVDLNKCEPWQLPGRAKMGEKEWYFFSLRDRKYPTGLRTNRATEAGYWKATGKDREIYSSKTCALIGMKKTLVFYKGRAPKGEKSNWVMHEYRLEGKFSYHFISRSSKDEWVISRVFQKTGLLSTGAAGAGAIVSGSNGTGTSKKTKIPSTISRNYQEQPSSPSSVSLPPLLGYTDSSCSYDGHSTNTTVTATGITEHVSCFSTATTTNTTTTDLGLDVNVDSFNHFPPPVFDPLPRFVSRNVSNLSNFRSFQDNQFPYFGSSSSASTMTSSVHLPSSQSGGSGVSGMNYWLQATAEENETKAGVLQAGLDCIWNY |
| ChCUC3 | ACL14365.1 | Cardamine hirsuta | MMLAVEDVLSELAGEERNERGLPPGFRFHPTDEELISFYLASKVFDGGLCGIHITEVDLNRCEPWELPEMAKMGEKEWYFYSLRDRKYPTGLRTNRATTAGYWKATGKDKEVFGSGGGQLVGMKKTLVFYKGRAPRGLKTKWVMHEYRLETDLSHRHSCKEEWVICRVFNKTGDRKNVGVHSQISCLHNHSLSTYHHHHHETLPPLLEPSKTISNFPSLLYDDHTHQNHNNNLFHGSSGHHHIDELKALINPVVSQLNGIIFSPGNNNNVDDEDDFNLGVKTEPFLNGGSNELDVRDYLENPLFHEVGYGLLGVSSAPGPLHMLLDSPCPLGFQL |
| OsNAM | EAZ00836.1 | Oryza sativa | MERCSVLGLGGGGGGGGRLDGELPPGFRFHPTDEELITYYLLRKVVDGSFNGRAIAEIDLNKCEPWELPEKAKMGEKEWYFYSLRDRKYPTGLRTNRATGAGYWKATGKDREIRSARTGALVGMKKTLVFYRGRAPKGQKTQWVMHEYRLDGTYAYHFLSSSTRDEWVIARIFTKPGVFPVVRKGRLGISGGGGDTSCFSDSTSASVGGGGGTSASSALRAPLAEASLFAAAAAPAVDGADSSNYGGGGGGGSATATANLVTGLELVPCFSTTAHMDASFGTGQYNPAPLAVEPPPPPPAFFPSLRSLQENLQLPLFLSGGMQAGVSSQPLSGSGAFHWQSGMDVKVEGAVGRAPPQMAVGPGQLDGAFAWGF |
| OsCUC3 | NP_001062212.1 | Oryza sativa | MPTTEILQHYSVVSQIKSHGKGIASEFPSALASWSADQISTDGAANLAGKLKQARSQIKNWTKNRTSCRFLDNDCKFVIDLFDFLEELRELSAPERLLRQMVQDKFTQYKLMQASYWKQRGKVKKIRLGIDNTHFFKAHATQNHRRTFIRSIKLTDMEVSEHSDKATTMFSYYNSILGASTETSWSFDLHTIYHGCAMANADELVQPFSEQEIFQAIKHMDKNSAPGPDGFGPGFFQAAWAMIKPDILHLLQSFYDETADMERINRAFIVLIPKPGKTNTPDGYRPISLQNCSVKIIAKVMANRMQRQLPPLIDLDQSGFLKREKHLGELHLCKILQARGFPFLWRSWTNRLLQTSKSAIMINGVPGNWINCKRGVRQGDPMSPYLFILVADVLQKLIRHSGEIKHPIYPDQPCATLQYADDTIIICRATEQDLAALKTCLNHFAAATGLHINFSKSTLVTMHVPDEVTTALANILQCKTDSSWGRWIWQEHSGSALFCDNQLGPHWDSLSTLLPILQRLTRVQVGDGTRTSFWHDCWYGSSTFKDRFAPLFSHALNREATVAVFLSKPIEDQFAPRLSSTAETQLARLREMLQNFYLSNSTDLCPSRDAPGILRTKFIYSSTHMGLPLCKNWRFIWDNRAPPRVQFFAWLLAKDRLPTKANLHKKNIVPTAGCIVCNCADETATHLFLQCQFAQEFWRALRTSVVSNVQDLADLVAPCHLPVKHFQVFFLLCFWGLWNHRHDVVFRGLPNSRTRNLQSLKPAESQAAGQPPEVWYACVAWAQLCRDIADNRTKPRSAAFEKSRNQPAIEHACMQGKNGFCVRGTSDSEMHHHSATMGDALWEMLGEEMAAAAAAAGEHGLPPGFRFHPTDEELVTFYLAAKVFNGACCGGVDIAEVDLNRCEPWELPEAARMGEKEWYFFSLRDRKYPTGLRTNRATGAGYWKATGKDREVVAAAAAGGALIGMKKTLVFYKGRAPRGEKTKWVLHEYRLDGDFAAARRSTKEEWVICRIFHKVGDQYSKLMMMKSPASYYLPVSHHHPSSIFHDLPPVPFPNPSLVPFHHDLPTSFHPPLLQHSHANSKNSSSNNGGFVFPNEPNTTNSSDNHISCNGAMAAAAAAAFPSFSCASTVTGKGGPPAQLGVNAGQQEPPPPTWMDAYLQHSGFIYEMGPPAVPRGA |
| ZmNAM1 | CAH56057.1 | Zea mays | MERFGLDGGGGGGELPPGFRFHPTDEELITYYLLRKAVDGSFCGRAIAEIDLNKCEPWELPGKAKMGEKEWYFYSLRDRKYPTGLRTNRATVAGYWKATGKDREIRSGRTGALVGMKKTLVFYRGRAPKGQKTHWVMHEYRLEGAYAYHFLPSSTRQDEWVIARVFQKPGEVPPAARKHRLGALSSTTGTAAGDSCFSDSTSASIGGASSSSTPGPLFASAAAAVANAGAADGDTSSYCGGAANHGNLVTGRELVPCFSTATINGPLVAAALGIGQPYNAAPLPFEQQPPPPAFLPSLRSLQDNLQLPPFLSAGGLGGGGALHWLPAGGMEVKVEGRSAPPQMAVGPGQLDGAFGWSF |
| ZmNAM2 | CAH56058.1 | Zea mays | MERLGVGVGVGELPPGFRFHPTDEELITYYLLCKAVDGGFCGGRAIAEIDLNKCEPWELPGKAKMGEKEWYFYCLRDRKYPTGLRTNRATAAGYWKATGKDREVRSGRSGALVGMKKTLVFYRGRAPRGQKTRWVMHEYRLDGTYAYHFLPGSTRDEWVIARVFQKPGEVPCGRKHRLGGPSAAAGDSCFSDSTTSASIGGGGGGGASASSRPLLTVTDTSSPSLFVANANAAASNNNGNPVTGRELVPCFSTTASPLEAAALGVVGHPYNAAPLRLGLDFEAPSPGFVVPNLRSLQVQDDGGLPLFLSAAAGGGMSSATLGIMGSLGGSLHCPPHAGMDVVKVEGRAAPPQMAVGPGLLDGAFAWGF |
| ZmCUC3 | CAH56059.1 | Zea mays | MAAAGGEHGLPPGFRFHPTDEELVTFYLAAKVFNGACCGIDIAEVDLNRCEPWELPDAARMGEREWYFFSLRDRKYPTGLRTNRATGAGYWKATGKDREVLNAATGALLGMKKTLVFYKGRAPRGEKTKWVLHEYRLDGDFAAARRPCKEEWVICRILHKAGDQYSKLMMVKSPYYLPMAMDPSSFCFQEDPTGHPLPNPSGCTPFHHGHPHHSMQPPPPLPPSNHAGKAVFTGAAAACCMQQEPADGSNSAVLPMPPFPPFTPIVAGKPAAPAPPPQVVNAGPQEPPPPTWLEAYLQHTGGILYEMGPTAAPRGA |
| SlNAM | ACL14371.1 | Solanum lycopersicum | MEIYHQMQFDCGDPHLPPGFRFHPTDEELITYYLLKKVLDCNFTARAIAEVDLNKCEPWELPGKAKMGEKEWYFFSLRDRKYPTGLRTNRATEAGYWKATGKDREIFSSKTCALVGMKKTLVFYRGRAPKGEKSNWVMHEYRLDGKFAYHYISRSSKDEWVISRVFQKSTGSNGAATSTGGGKKRLSSSINMYQEVSSPSSVSHLPPLLDSSPYSTTATSAAAIVIGDRDRDHSFKKEHVPCFSTTATATITAQSLTFDPTSVFDISSNTLHALQPTPSFASILDSSPSNFTNYTRNSTFPSLRSLHENLQLPLFSGGTSAMHGGFSNPMVNWTVPETQKVEQSELDCMWSY |
| VvNAM1 | XP_002282655.1 | Vitis vinifera | MDAYHHFDNSDAHLPPGFRFHPTDEELITYYLIKKVLDSNFTGRAIAEVDLNKCEPWELPEKAKMGEKEWYFFSLRDRKYPTGLRTNRATEAGYWKATGKDREIYSSKTCSLVGMKKTLVFYRGRAPKGEKSNWVMHEYRLEGKFAYHYLSRSSKDEWVISRVFQKSGSSGGGGATGGKKARLSSTVNLYPEVSSPSSASLPPLLDVSPYAGTSAAAAVNDRESCSYDGGESSNNSNARDQHVPWFSTIAAAAAAAAANSFNAHHQPPPFDLAPPSIIGSIDPSRFPRNGAVPAFPNLRSLQENLHLPFFFSQVAPPIPSSGDPSTEMGITNSAGNWPAPENQKMDNGRLPMGATELDCMWSY |
| VvNAM2 | XP_002280812.1 | Vitis vinifera | MEEERKEETLPPGFRFHPTDEELITCYLINKISDATFTGRAIADVDLNKCEPWELPGKAKMGEKEWYFFSLRDRKYPTGVRTNRATNTGYWKTTGKDKEIFNSVTSELVGMKKTLVFYRGRAPRGEKTNWVMHEYRIHSKSAFRTSKDEWVVCRVFQKSAAGKKYPSNQSRGMNPYSLDIGPSVMPPPMLQADSSQFPMGRNYVSNAELAELTRVLRGGSTGGLNLPIQSQLNYPLGGGCFTISGLNLNLGGTSTQPVLRPNSLPQPMQMNQQDHMMTSPMLTSGSIPTDQTGYGAEVNNGNGHNSRFMNMVDHCVDLDNYWPPY |
| VvNAM3 | XP_002276293.2 | Vitis vinifera | MQQAANQMHEKMEESLPPGFRFHPTDEELITYYLTPKVSNTNFASRAIADVDLNKCEPWDLPAKASMGEKEWYFFSLRDRKYPTGIRTNRATEAGYWKTTGKDKEIYRAGILVGMKKTLVFYKGRAPKGEKSNWVMHEYRLETKLSFKPKKEEWVVCRVFKKSSAVKKPHQPAPSSLPSLESPCDTNTIVSEFGDIEFPNMNSIANSSSGFSNINSAQSYNTSTDDNLNMNMNMNMNMNWAAAREAASLPSLPWSSSLLSPNLQMNSLLIKALQLGSYRPREATSTENYSFLPQGISNFGTDFISNFQASSSKVLDSLHQQQQQQQQEQPFNLDSINWFGHMSRSNSLNYLGKHSTSRLINSHRTAPQHKQAAGLSSCHMFLTPGPAPFLVNVLKTHGHHGSQQQSILCHTITHTKQLTQLLTSSAALLAACHSGLLPGASTAAPLCSVASTGQWLPSSICSAQPTCRRRQDSAKENPWDGLMGMVGDNGYEEVNRKGHVWPCKGGGDRHEGRASMVMGIRRTMSLAWRLMHGMIPHGA |
| VvCUC3 | XP_002273222.1 | Vitis vinifera | MLAMEEVLCELSREDINEQGLPPGFRFHPTDEELITFYLASKVFNGSFCGVEIAEVDLNRCEPWELPDVAKMGEREWYFFSLRDRKYPTGLRTNRATGAGYWKATGKDREVHSASSGALLGMKKTLVFYKGRAPRGEKTKWVMHEYRLDGDFSYRHTCKEEWVICRIFHKTGEKKNPMFQGQAYLLGSSAAAAVATSSLPALLESQTTLLESQSHPTMQGGISSSFLVHHHHDQESNELKALINPVLSQSPLAFPINSGFQSCSFSTTPTTNIPNTNNINSTTGNNNPSTSILFKSLLSHQECSLKEQTTIPKQCKTEANFSHFQLPDANMHWVDRMNSNLHQNPLFFEMDYCSGGVLGFTAATATGGGGGGGGASAAATSAAVASAAETVHEMSTSIAFNRAGFQMMVDFPIRVPGGESWPLDP |
| BoCUC2 | HQ703968 | Brassica oleracea | DHGGDSQYLPPGFRFHPTDEELITHYLLRKVIEGCFSSRAIAEVDLNKSEPWQLPGKAKMGEKEWYFFSLRDRKYPTGLRTNRATEAGYWKATGKDREIYSSKTCALVGMKKTLVFYKGRAPKGEKSSWVMHEYRLEGKFSYHFISRSSKDEWVISRVFKKTGLATTGASAGASISVSNCTGTSKKTKIPSNISTNYREQPSSPSSVSLPPLLDPTTTLGYTDSSWSYDSRSTNTPVITTAIT |
| BoCUC3 | HQ703970 | Brassica oleracea | NDRGLPPGFRFHPTDEELITFYLASKVFHGGLCGIHIAEVDLNRCEPWELPEMAKMGEREWYFYSLRDRKYPTGLRTNRATTAGYWKATGKDKEVFAGGGSGGGALVGMKKTLVFYKGRAPRGLKTKWVMHEYRLETDLSHRHTCKEEWVICRVFNKTGDRKNVGIHNQISYLHNTSLSTTHQQRNHNHYHHLEILPPLLEPSKTLTNFPSLLYDDTHQNYNNNLLHGSSGHNVDEFKTLINPAVSQLNGVIFSPEISNYNNEDDNNFGIKTEQYSNGGNNDLDVRDYLDNPFCQEAGYGLLGLSSSPGPLM |
References
- Fleming, A.J. The control of leaf development. New Phytol. 2005, 166, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Holtan, H.E.; Hake, S. Quantitative Trait Analysis of Leaf Dissection in Tomato Using Lycopersicon pennellii Segmental Introgression Lines. Genetics 2003, 165, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Barkoulas, M.; Galinha, C.; Grigg, S.P.; Tsiantis, M. From genes to shape: Regulatory interactions in leaf development. Curr. Opin. Plant Biol. 2007, 10, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Nikovics, K.; Blein, T.; Peaucelle, A.; Ishida, T.; Morin, H.; Aida, M.; Laufs, P. The Balance between the MIR164A and CUC2 Genes Controls Leaf Margin Serration in Arabidopsis. Plant Cell 2006, 18, 2929–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukaya, H. Leaf shape: Genetic controls and environmental factors. Int. J. Dev. Biol. 2005, 49, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoln, C.; Long, J.; Yamaguchi, J.; Serikawa, K.; Hake, S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 1994, 6, 1859–1876. [Google Scholar]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Chuck, G.; Lincoln, C.; Hake, S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 1996, 8, 1277–1289. [Google Scholar]
- Pautot, V.; Dockx, J.; Hamant, O.; Kronenberger, J.; Grandjean, O.; Jublot, D.; Traas, J. KNAT2: Evidence for a Link between Knotted-Like Genes and Carpel Development. Plant Cell 2001, 13, 1719–1734. [Google Scholar] [CrossRef] [Green Version]
- Piazza, P.; Bailey, C.D.; Cartolano, M.; Krieger, J.; Cao, J.; Ossowski, S.; Schneeberger, K.; He, F.; de Meaux, J.; Hall, N.; et al. Arabidopsis thaliana leaf form evolved via loss of knox expression in leaves in association with a selective sweep. Curr. Biol. 2010, 20, 2223–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P.; et al. Comprehensive Analysis of NAC Family Genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Vroemen, C.W.; Mordhorst, A.P.; Albrecht, C.; Kwaaitaal, M.A.; de Vries, S.C. The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell 2003, 15, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Werr, W. Pattern formation in the monocot embryo as revealed by NAM and CUC3 orthologues from Zea mays L. Plant Mol. Biol. 2005, 58, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Hasson, A.; Plessis, A.; Blein, T.; Adroher, B.; Grigg, S.; Tsiantis, M.; Boudaoud, A.; Damerval, C.; Laufs, P. Evolution and diverse roles of the cup-shaped cotyledon genes in Arabidopsis leaf development. Plant Cell 2011, 23, 54–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibara, K.; Karim, M.R.; Takada, S.; Taoka, K.; Furutani, M.; Aida, M.; Tasaka, M. Arabidopsis CUP-SHAPED COTYLEDON3 regulates postembryonic shoot meristem and organ boundary formation. Plant Cell 2006, 18, 2946–2957. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.; Greb, T.; Peaucelle, A.; Blein, T.; Laufs, P.; Theres, K. Interplay of miR164, CUP-SHAPED COTYLEDON genes and LATERAL SUPPRESSOR controls axillary meristem formation in Arabidopsis thaliana. Plant J. 2008, 55, 65–76. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef] [Green Version]
- Hibara, K.; Takada, S.; Tasaka, M. CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation. Plant J. 2003, 36, 687–696. [Google Scholar] [CrossRef]
- Takada, S.; Hibara, K.; Ishida, T.; Tasaka, M. The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 2001, 128, 1127–1135. [Google Scholar] [CrossRef]
- Souer, E.; van Houwelingen, A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Weir, I.; Lu, J.; Cook, H.; Causier, B.; Schwarz-Sommer, Z.; Davies, B. CUPULIFORMIS establishes lateral organ boundaries in Antirrhinum. Development 2004, 131, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Blein, T.; Pulido, A.; Vialette-Guiraud, A.; Nikovics, K.; Morin, H.; Hay, A.; Johansen, I.E.; Tsiantis, M.; Laufs, P. A conserved molecular framework for compound leaf development. Science 2008, 322, 1835–1839. [Google Scholar] [CrossRef] [Green Version]
- Berger, Y.; Harpaz-Saad, S.; Brand, A.; Melnik, H.; Sirding, N.; Alvarez, J.P.; Zinder, M.; Samach, A.; Eshed, Y.; Ori, N. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 2009, 136, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Li, Y.; Liu, T.; Duan, W.; Huang, Z.; Wang, L.; Tan, H.; Hou, X. Genes associated with agronomic traits in non-heading Chinese cabbage identified by expression profiling. BMC Plant Biol. 2014, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Mara, C.; Grigorova, B.; Liu, Z. Floral-dip transformation of Arabidopsis thaliana to examine pTSO2::β-glucuronidase reporter gene expression. J. Vis. Exp. 2010, 40, 1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, N.; Savolainen, V.; Chase, M.W. Evolution of the angiosperms: Calibrating the family tree. Proc. Biol. Sci. 2001, 268, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Lai, K.N.; Tai, P.Y.; Li, W.H. Rates of nucleotide substitution in angiosperm mitochondrial DNA sequences and dates of divergence between Brassica and other angiosperm lineages. J. Mol. Evol. 1999, 48, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, T.; Coulibaly, S.; Sadowski, J.; Quiros, C.F. Variation and phylogenetic utility of the Arabidopsis thaliana Rps2 homolog in various species of the tribe Brassiceae. Mol. Phylogenet. Evol. 2000, 16, 440–448. [Google Scholar] [CrossRef]
- Galbiati, F.; Sinha Roy, D.; Simonini, S.; Cucinotta, M.; Ceccato, L.; Cuesta, C.; Simaskova, M.; Benkova, E.; Kamiuchi, Y.; Aida, M.; et al. An integrative model of the control of ovule primordia formation. Plant J. 2013, 76, 446–455. [Google Scholar] [CrossRef]
- Wen, S.; Li, J.; Hao, Z.; Wei, L.; Ma, J.; Zong, Y.; Li, H. Overexpression of the LcCUC2-like gene in Arabidopsis thaliana alters the cotyledon morphology and increases rosette leaf number. Peer J. 2022, 2, e12615. [Google Scholar] [CrossRef]
- Barro-Trastoy, D.; Gomez, M.D.; Blanco-Touriñán, N.; Tornero, P.; Perez-Amador, M.A. Gibberellins regulate ovule number through a DELLA-CUC2 complex in Arabidopsis. Plant J. 2022, 110, 43–57. [Google Scholar] [CrossRef]
- Vogel, S. Leaves in the lowest and highest winds: Temperature, force and shape. New Phytol. 2009, 183, 13–26. [Google Scholar] [CrossRef]
- Sisó, S.; Camarero, J.; Gil-Pelegrín, E. Relationship between hydraulic resistance and leaf morphology in broadleaf Quercus species: A new interpretation of leaf lobation. Trees 2001, 15, 341–345. [Google Scholar] [CrossRef]
- Semchenko, M.; Zobel, K. The role of leaf lobation in elongation responses to shade in the rosette-forming forb Serratula tinctoria (Asteraceae). Ann. Bot. 2007, 100, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugarny, A.; Gonalves, B.; Arnaud, N.; Laufs, P. Cuc transcription factors: To the meristem and beyond. In Plant Transcription Factors; Academic Press: Cambridge, MA, USA, 2016; pp. 229–247. [Google Scholar]
- Kamiuchi, Y.; Yamamoto, K.; Furutani, M.; Tasaka, M.; Aida, M. The CUC1 and CUC2 genes promote carpel margin meristemformation during Arabidopsis gynoecium development. Front. Plant Sci. 2014, 5, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, M.A.; Ishida, T.; Smyth, D.R.; Tasaka, M.; Aida, M. Interactions of CUP-SHAPED COTYLEDON and SAPTULA genes control carpel margin development in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Wei, W.; Li, Y.; Kan, L.; Wang, F.; Zhang, X.; Li, F.; Liu, Z.; Kang, C. Conserved and novel roles of miR164-CUC2 regulatory module in specifying leaf and floral organ morphology in strawberry. New Phytol. 2019, 224, 480–492. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, T.; Ma, Y.; Wang, N.; Wang, W.; Tang, J.; Zhang, C.; Hou, X.; Hou, H. Ectopic Expression of BcCUC2 Involved in Sculpting the Leaf Margin Serration in Arabidopsis thaliana. Genes 2023, 14, 1272. https://doi.org/10.3390/genes14061272
Li W, Wang T, Ma Y, Wang N, Wang W, Tang J, Zhang C, Hou X, Hou H. Ectopic Expression of BcCUC2 Involved in Sculpting the Leaf Margin Serration in Arabidopsis thaliana. Genes. 2023; 14(6):1272. https://doi.org/10.3390/genes14061272
Chicago/Turabian StyleLi, Wanqi, Tongtong Wang, Yu Ma, Nan Wang, Wenjing Wang, Jun Tang, Changwei Zhang, Xilin Hou, and Hualan Hou. 2023. "Ectopic Expression of BcCUC2 Involved in Sculpting the Leaf Margin Serration in Arabidopsis thaliana" Genes 14, no. 6: 1272. https://doi.org/10.3390/genes14061272