- 1Inner Mongolia Key Laboratory of Characteristic Geoherbs Resources Protection and Utilization, Baotou Medical College, Baotou, China
- 2Trauma Orthopedic, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
- 3Department of Pharmacy, Inner Mongolia Medical University, Hohhot, China
- 4Inner Mongolia Institute of Traditional Chinese Medicine, Hohhot, China
- 5Key Laboratory of Resourceology of Chinese Medicinal Materials, Baotou, China
- 6Inner Mongolia Engineering Research Center of The Planting and Development of Astragalus Membranaceus of the Geoherbs, Baotou, China
Odontites vulgaris Moench has the effect of clearing away heat, detoxification, dispelling wind, and clearing dampness. In this study, the potential anti-inflammatory compounds of O. vulgaris were investigated using ultra-high-performance liquid chromatography–mass spectrometry (UPLC–MS/MS) combined with the network pharmacology approach and further confirmed on an LPS-activated RAW 264.7 macrophage model. Monomer compounds were prepared from the active fraction using modern advanced separation and purification methods. UPLC–Q-Exactive HRMS was used to identify the chemical compounds in the active fractions of O. vulgaris. D-mannitol, geniposidic acid, salidroside, shanzhiside methyl ester, eleutheroside B, geniposide, 7,8-dihydroxycoumarin, gardoside methyl ester, arenarioside, vanillic acid, p-hydroxy-cinnamic acid, melampyroside, syringaresinol, tricin, and diosmetin were isolated from O. vulgaris for the first time. A compound database of O. vulgaris was established based on the existing literature to predict the mechanism of O. vulgaris in the treatment of rheumatoid arthritis. The results suggest that the PI3K-Akt pathway mediates O. vulgaris and deserves more attention in the treatment of RA. Finally, the anti-rheumatoid arthritis effects of the four target compounds were validated with the decreased levels of NO, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophage cells treated with LPS. The present study explored the potential targets and signaling pathways of O. vulgaris in the treatment of RA, which may help to illustrate the mechanisms involved in the action of O. vulgaris and may provide a better understanding of the relationship between O. vulgaris and RA. This study provides novel insights into the development of new drugs and utilization of Mongolian traditional Chinese medicine resources.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by persistent synovial hyperplasia, inflammatory infiltration, pannus formation, and bone erosion, which may lead to joint deformity, disability, and death (Li et al., 2019a; Li et al., 2019b; Guo et al., 2020). Thus far, pain relief and reduction of inflammation have been considered the main therapeutic strategies for RA. With a high disability rate, RA poses a serious threat to human health and is a prevalent, globally distributed incurable diseases. Without timely treatment, the incidence of disability in patients with RA is reported to be approximately 70% (Li and Gu, 2020). Non-steroidal anti-inflammatory drugs are commonly used for the treatment of such patients. However, long-term use of these drugs can cause considerable side effects; for example, gastrointestinal mucosal injury and bleeding occur more frequently in patients with long-term use of these drugs than in those with gastrointestinal diseases (Vetal et al., 2013; Li et al., 2018). Biological blockers that suppress inflammation, such as that mediated by anti-tumor necrosis factor-α, may lead to a higher risk of infection owing to their role in weakening the immune system (Wang et al., 2020a). The interaction of a drug with an unintended target may be a source of adverse drug reactions and side effects. Therefore, there is an urgent need to find safe and effective drugs for the treatment of RA with fewer side effects than those currently used. In recent years, owing to the low level of ethnopharmacological adverse reactions, Ethnopharmacological Mongolian medicine has attracted attention as an alternative treatment for inflammation (Wang et al., 2020b). These Mongolian medicinal treatments exert anti-inflammatory effects through the NF-κB, MAPK, PI3K-Akt, arachidonic acid metabolism, inflammasome, and other pathways (Mao et al., 2019).
In Mongolian medicine, RA is often called “Xieri Wusu” or “Tuolai disease” (Wang et al., 2018) and is a “Taolai” disease (Bai et al., 2015). “Basaga,” with its name derived from the transliteration of a Sanskrit word, is often used in Mongolian medicine with remarkable curative effects, such as cooling blood, reducing itching, detoxification, and diuresis (Zhao et al., 2017). According to traditional Chinese medicine, RA is a disease caused by wind, cold, dampness, heat, and other evil gases that close and obstruct meridians and collateralization, leading to pain in the limbs, bones, and joints caused by poor flexion and extension, stiffness, and deformation (Liu et al., 2015). Odontites vulgaris Moench (Figure 1) is a commonly used Mongolian medicine variety of “Basaga.” It helps in clearing away heat, detoxification, dispelling wind, and clearing dampness. The primary compounds present in O. vulgaris are phenylethanol glycosides, iridoids, and flavonoids. Some compounds have been shown to have certain anti-inflammatory effects (Gong et al., 2020; Nigro et al., 2020; Wang et al., 2020d; Cicek et al., 2021; Tian et al., 2021). Through interviews with the local populace and hospital personnel in Inner Mongolia, we found that O. vulgaris is often used to treat RA; therefore, we conducted an in-depth study on the effects of O. vulgaris on RA. A flowchart of this process is shown in Figure 2.
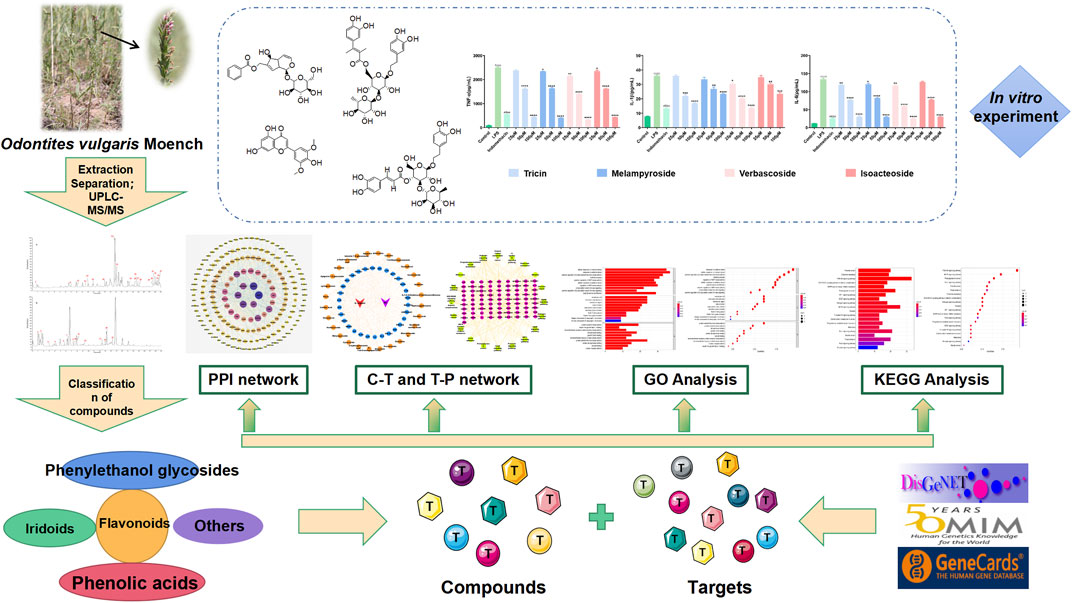
FIGURE 2. Flow chart of network pharmacological and experimental studies on the effect of O.vulgaris in the treatment of RA.
At present, multi-compound, multi-target therapy has been reported to be more effective and less toxic than the use of traditional single-target drugs (Zhang et al., 2013a). Network pharmacology is a new, promising, and low-cost drug development method based on bioinformatics, systems biology, and multi-pharmacology. For Mongolian traditional Chinese medicines with complex compositions and unclear mechanisms of action, especially medicine varieties with weak foundations in research, network pharmacology not only serves as an effective tool for studying the mechanism of action, but also provides a basis for designing in vivo and in vitro experiments (Zhang et al., 2013a; Zheng et al., 2014; Zhou et al., 2016; Jiang et al., 2019).
To the best of our knowledge, this study is the first to use UPLC–MS/MS technology to study the chemical compounds produced by O. vulgaris. We isolated 17 compounds, of which 15 compounds were newly reported. We used network pharmacology combined with computer assisted techniques-molecular docking to predict the target and action pathways of O. vulgaris in RA treatment and detected its target compounds from the level of inflammatory factors in vitro. This study explored potential targets and signaling pathways for the treatment of RA with O. vulgaris, which may help to illustrate the mechanisms of action involved in RA. Moreover, our results can promote the development and utilization of Mongolian traditional Chinese medicine resources.
Materials and Methods
Plant Materials
O. vulgaris was collected from the Xilinguole League of Inner Mongolia Autonomous Region in 2018 and identified as Odontites vulgaris Moench by a specialist. The specimen (specimen number: 152500180629025LY) was kept in the herbarium of Baotou Medical College.
Chemical Materials
RAW 264.7 cells were purchased from the Cell Bank at the China Academy of Science (Shanghai, China). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline, penicillin, and streptomycin were purchased from Gibco (New York, United States). Dimethyl sulfoxide (DMSO) and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sangon Biotech (Shanghai, China). Lipopolysaccharides (LPS) and indomethacin were obtained from Sigma-Aldrich (United States). The nitric oxide (NO) kit was purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were obtained from Beijing Solarbio Science & Technology Co. Ltd (China). Ultrapure water was obtained from Gen Pure (Thermo Fisher Scientific, Waltham, MA, United States). Acetonitrile (HPLC grade) was purchased from Thermo Fisher Scientific. All reagents were of analytical grade. Reference compounds for the 15 analytes were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Purified water was obtained using a Milli-Q system (Millipore, Burlington, MA, United States).
Extraction and Processing
Whole dried O.vulgaris (6.04 kg) powder was successively extracted with 70% ethanol; approximately 1.2 kg of the extract was obtained. The extract was dissolved in water and extracted with petroleum ether, ethyl acetate, and n-butanol to obtain 17.56, 73, and 259 g of dry extracts, respectively. The aqueous phase contained 755 g of residue.
Screening of the Protective Effects of the O. vulgaris Extracts on LPS-Induced Inflammation in RAW 264.7 Cells.
Cell Culture
RAW 264.7 cells were cultured in DMEM supplemented with 10% FBS and a mixture of penicillin and streptomycin. The cells were incubated at 5% CO2 and 37°C.
Effect of the Crude Extract of O. vulgaris on Cell Viability
Logarithmic phase RAW 264.7 cells seeded in 96-well plates at a density of 1 × 105 cells/ml were cultured at 37°C in a 5% CO2 incubator for 24 h. After adherence of the cells to the plates, a series of different concentrations (25, 50, 100, 200, and 400 μg/ml) of the O.vulgaris extracts (water, n-butanol, ethyl acetate, and petroleum ether) was added. The control group (without the extract) was set at the same time. Five parallel wells were used in each group. After 48 h of culture, 10 μl of the freshly prepared 5 mg/ml MTT solution was added to each well, and the culture was incubated at 37°C for 4 h in the dark. The supernatant was discarded, and 150 µl of dimethyl sulfoxide (DMSO) was added to each well. After oscillation for 10 min, the absorbance (OD 570 nm) of each well was measured using a microplate analyzer (Thermo Scientific Multiskan FC, Thermo Fisher Scientific).
Determination of Indomethacin Concentration
Logarithmic phase RAW 264.7 cells seeded in 24-well plates at a density of 5 × 105 cells/ml were placed in a 37°C 5% CO2 incubator for 24 h. After adherence to the plate, the cells were randomly divided into the control group, model group (LPS), and positive drug group (indomethacin group). Each group was placed in parallel holes. The cells were cultured with LPS (1 μg/ml) for 4 h, with the positive drug group being administered indomethacin solutions of different concentrations to co-interact with LPS cells for 20 h at the following concentrations: 6.25, 12.5, 25, 50, and 100 µM. The cell supernatant of each group was collected after culture, and NO release in the cell supernatant was detected according to the manufacturer’s instructions. The concentration of NO was determined using sodium nitrite as the control substance, and the 50% inhibition rate (i.e., the IC50 value) was calculated according to the concentration of NO. Absorbance at 550 nm was determined using a microplate analyzer.
Cell Viability Assay and Protective Effects of the Extracts on LPS-Treated RAW 264.7 Cells
Single-cell suspension RAW 264.7 cells were seeded at a density of 5 × 105 cells/ml in 24-well plates and placed at 37°C in a 5% CO2 incubator for 24 h until the logarithmic phase was reached. The group demarcation based on treatment in this study was as follows: control group with normal cell culture for 24 h without any treatment; inflammation model group with cells stimulated using 1 μg/ml LPS for 24 h; negative control group with cells treated using different extracts (alcohol, ethyl acetate, n-butanol, water, and petroleum ether fractions) of O.vulgaris at 400 μg/ml for 20 h; and the experimental group with cells stimulated with 1 μg/ml LPS for 4 h and different concentrations (12.5, 25, 50, 100, and 200 μg/ml) of O.vulgaris extracts added to the cells for 20 h. The cell supernatant of each group was collected after culture, and NO release in the cell supernatant was detected according to the Griess test described in the NO kit. Absorbance was measured at 550 nm (Gong et al., 2020).
Chemical Isolation of Pure Compounds
The n-butanol extract (80 g) was separated on a D101 macroporous resin column (ethanol-water, 70:30), and eight fractions were obtained. Thin-layer chromatography was used to analyze the compounds. Three main fractions (Frs. A1–A3) were ultimately obtained by combining the fractions with similar polarity and compound composition. Separation was carried out on an octadecylsilyl (ODS) column (separation principle: solute polarity difference, 800 g; methanol: H2O, 30:70, 60:40, and 90:10; 2.5, 2.5, and 4 L, v/v). After separation and purification, 7.6 mg of compound 1 and 2.4 mg of compound 2 were obtained. Fr. A2 was analyzed using atmospheric pressure silica gel column chromatography and confirmed using Sephadex gel column (Sephadex LH-20) chromatography. We obtained 5.3 mg of compound 3. Fr. A3 was further separated on an ODS column (280 g; methanol: H2O, 40:60; 3 L, v/v) into three compounds (B1–B3). Fr. B1 was repeatedly eluted with Sephadex LH-20 (methanol: H2O, 45:55) to yield 25.4 mg of compound 4 and 6.4 mg of compound 5. Fr. B2 was separated on a silica gel column (methanol: H2O, 5:95–90:10) and eluted with methanol on a Sephadex LH-20 column to obtain 14 mg each of compounds 6 and 7. Compounds 8 (16.6 mg) and 9 (27.3 mg) were obtained from Fr. B3 using preparative HPLC. Compounds 10 (34.9 mg) and 11 (19.8 mg) were obtained from Fr. C4 by repeated elution with Sephadex LH-20 (methanol-water, 60:40).
Ethyl acetate extract (60 g) was prepared using a small pore resin coagulant gel column (MCI; separation principle: gel filtration and reverse-phase distribution, 700 g; ethanol: H2O, 30:70, 60:40, and 90:10; 2.5, 2.5, and 3 L, v/v) for chromatographic analysis, and nine fractions (D1–D9) were obtained. Compounds 12 (9 mg) and 13 (10 mg) were obtained using preparative liquid chromatography. Compounds 14 (34.0 mg) and 15 (22 mg) were obtained from Fr. D3–D5 using a silica gel column and a Sephadex LH-20 column. Compounds 16 (20.4 mg) and 17 (18.7 mg) were isolated from Fr. D8–D9 using RP-18, methanol-water gradient elution, Sephadex LH-20, and preparative HPLC.
UPLC–MS
Ultra-high-performance Liquid Chromatography–Mass Spectrometry (UPLC-MS/MS) System
The UPLC high-resolution MS Dionex Thermo Scientific UItimate 3,000 system connected with the Thermo Q Exactive focus machine was used in this study. For the analysis, the extracts of n-butanol and ethyl acetate were redissolved (1 mg/ml) in methanol and the sample was filtered through a 0.22 µm microporous membrane to obtain the test solution, and 10 µl of the filtered solution was injected into the instrument.
UPLC–MS/MS System and Operating Conditions
A C18 column (100 × 2.1 mm, 3 μm; Thermo Fisher Scientific) was used in the experiment. The mobile phase consisted of 0.1% formic acid aqueous solution (A), acetonitrile (B), gradient elution, 0–35 min, 95%–5% (A), 5–95% (B). The injection volume was 1 μl, flow rate was 0.3 ml/min, and column temperature was 25°C.
Mass spectrometry conditions were as follows: ion source HESI source, negative ion detection mode; sheath gas pressure, 206.84 kPa; auxiliary gas volume flow, 15.22 L/min; spray voltage, 3.5 kV; ion transmission tube temperature, 320°C; auxiliary gas temperature, 350°C; scanning mode full MS/dd-MS2, full MS resolution 70,000; and dd-MS2 resolution 17,500, scanning range m/z 100–1,500. For MS/MS, the collision energies were 20, 40, and 60 eV. All MS spectra were collected using the Xcalibur 3.0 software of the UPLC–Q-Exactive quadrupole electrostatic field orbit trap high-resolution mass spectrometer.
Data Processing
After the sample solution was analyzed and detected according to the above mass spectrum conditions, the Qualbrowser feature of the Xcalibur 3.0 software was used to complete data acquisition and processing, including the extraction of the ion chromatogram and fragmentation behavior information. The mass error of each main fragment ion was within 5 ppm. Full-scan mode high-resolution mass spectrometry data were analyzed using the Mass Frontier 7.0 software, and the possible structure formulae were deduced. The actual molecular weights of the detected compounds were then matched with the theoretical values. The Discoverer 2.1 (including mzCloud, Chinese medicine compound database, Chemspider, and Sci finder) database was used to search for the compounds, match the first and second mass spectra of the compounds, analyze the pyrolysis law, and identify the compounds.
Network Pharmacology
Prediction of O. vulgaris Compound Information
The compound database was established through literature retrieval and compound identification in Chinese and English databases (CNKI, Wanfang, PubMed). Compound information was collated in the PubMed database (Hao et al., 2013). The PubChem database constantly adds new data, and the SMILES or chemical structure formulae can be uploaded to the Swiss target prediction database (Daina et al., 2019).
Prediction of O. vulgaris Targets for RA Treatment
Using “rheumatoid arthritis” as the keyword, RA-related targets were identified in the OMIM, GeneCards, and DisgeNet databases. The TBtools software was used to match the targets related to the chemical compounds of O.vulgaris with the targets of RA. Additionally, a Venn diagram was drawn to obtain the potential therapeutic targets of the compounds of O.vulgaris for RA.
Construction and Analysis of the Protein-Protein Interaction Networks of the Target Proteins
To study the interaction between the target proteins in the treatment of RA by O.vulgaris, a PPI network was constructed using the String Version 11.0 platform. We set a score greater than 0.7 to indicate high confidence (Szklarczyk et al., 2017; Deng et al., 2019; Zhou et al., 2019), set the species as Homo sapiens (human), and hid the nodes that were disconnected from the network, leaving the rest of the parameters as default (Liu et al., 2019). The network analyzer function in the Cytoscape 3.7.1 software was used to study the PPI network topology attributes and draw the PPI network diagram.
Enrichment Analysis of the Target Using Genome Ontology and the Kyoto Encyclopedia of Genes and Genomes
The KEGG pathway and GO enrichment analyses of 198 targets of O.vulgaris in the Clusterprofiler software package were carried out using R Version 4.0.2, and the results were visualized.
Molecular Docking
To further investigate the possibility of interaction between the four selected compounds (verbascoside, isoacteoside, melampyroside, and tricin) and the ten key targets (AKT1, MAPK1, EGFR, HRAS, APP, HSP90AA1, PIK3CA, SRC, PIK3R1, and AR), the structure of the small molecule compounds downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/) was saved in SDF format. The Chem 3D software was used to convert the SDF file into a * Mol2 file, and the 3D structure of the target protein (PDB format) was downloaded from the PDB database (https://www.rcsb.org/) (Burley et al., 2017). The PyMol software was used to dehydrate the proteolytic enzyme and remove the active center of the original ligand. At the same time, hydrogenation, charge adding, and non-polar hydrogen merging were carried out using the AutoDock Tools 1.5.6 software. We used AutoDock Vina 1.1.2 for docking operations (Tanchuk et al., 2016). The receptor-ligand pairs were sorted and screened according to the binding free energy [Affinity (kcal/mol)]. Binding energy less than 0 indicated that the compound and protein could spontaneously bind and interact with each other. The lower the energy, the more stable the molecular conformation. Generally, a binding energy of ≤5.0 kcal/mol indicated a good binding effect. The PyMol 2.3.2 software was used for visualization.
Anti-Inflammatory Assay
Effects of the Target Compounds of O. vulgaris on Cell Viability
Logarithmic phase RAW 264.7 cells distributed as single-cells seeded in 96-well plates at a density of 1 × 105 cells/ml were placed in a 37°C 5% CO2 incubator for 24 h. After the cells adhered to the plate, different concentrations (6.25, 12.5, 25, 50, 100, and 200 μM) of the target compounds of O.vulgaris (isoacteoside, verbascoside, tricin, and melampyroside) were added to the culture. The normal control group (without the extract) was established at the same time. Five parallel wells were used for each group. After 48 h of culture, 10 μl of the freshly prepared 5 mg/ml MTT solution was added to each well and the culture was incubated at 37°C for 4 h in the dark. The supernatant was discarded, and 150 µl of DMSO was added to each well. After oscillation for 10 min, the absorbance (OD 570 nm) of each well was measured using a microplate analyzer.
Cell Viability Assay and the Protective Effects of Target Compounds of O. vulgaris on LPS-Treated RAW 264.7 Cells
We performed this analysis using the methods described in Chemical Materials and Extraction and Processing. The group demarcation based on treatment in this study was as follows: control group normal cell culture for 24 h without any treatment; inflammation model group with cells stimulated using 1 μg/ml LPS for 24 h; negative control group with cells treated using different target compounds (isoacteoside, verbascoside, tricin, and melampyroside) of O.vulgaris at 100 μM for 20 h; experimental group with cells stimulated using 1 μg/ml LPS for 4 h and different concentrations (6.25, 12.5, 25, 50, and 100 μM) of different target compounds (isoacteoside, verbascoside, tricin, and melampyroside) of O.vulgaris for 20 h. Three parallel wells were set in each group. The cell supernatant of each group was collected after culture, and NO release in the cell supernatant was detected according to the Griess test described in the NO kit. Then, the absorbance was measured at 550 nm (Gong et al., 2020).
Enzyme-Linked Immunosorbent Assay
Logarithmic phase RAW 264.7 cells made into single-cell suspension cells under the scratch seeded in a 6-well plate at a density of 5 × 105 cells/ml were placed at 37°C in a 5% CO2 incubator for 24 h. The group demarcation based on treatment in this study was as follows: control group with normal culture without any treatment; inflammation model group with cells stimulated with 1 μg/ml LPS for 24 h; negative control group with cells treated with different target compounds (isoacteoside, verbascoside, tricin, and melampyroside) of O.vulgaris at 100 μM for 20 h; experimental group with cells stimulated using 1 μg/ml LPS for 4 h and different concentrations (25, 50, 100 μM) with different target compounds (isoacteoside, verbascoside, tricin, and melampyroside) of O.vulgaris for 20 h. Three parallel wells were set in each group. The supernatant of each group was collected, centrifuged for 5 min, and the supernatant was carefully removed for storage. The expression levels of TNF-α, IL-6, and IL-1β were detected using an ELISA kit.
Statistical Analysis
All data were analyzed using SPSS 25.0, and the results were expressed as mean ± standard deviation (SD) of the three independent experiments. Differences were considered statistically significant if p < 0.05.
Results
Screening of the Protective Effects of O. vulgaris Extracts on LPS-Induced Inflammation in RAW 264.7 Cells
Determination of Indomethacin Concentration
The results showed that indomethacin could significantly inhibit the content of NO (p < 0.0001), and the inhibitory effect was proportional to the concentration of indomethacin (i.e., the higher the concentration, the stronger the inhibitory effect). The IC50 value of indomethacin was 58.82 μM in linear regression analysis. Therefore, 60 μM indomethacin was selected as the positive drug concentration for subsequent experiments (Figure 3).
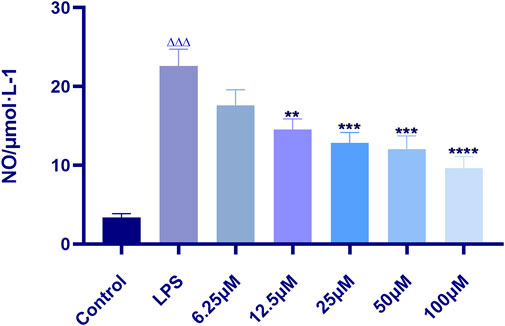
FIGURE 3. Effect of indomethacin on NO production in RAW 264.7 induced by LPS. Data are presented as means ± SD, n = 5. ** means p < 0.01, *** means p < 0.001, **** means p < 0.0001 compared with LPS group. △△△ means p < 0.001 compared with control group.
Effects of the Crude Extracts of O. vulgaris on Cell Viability
The effects of different polar fractions (alcohol, ethyl acetate, petroleum ether, water, and n-butanol) on the proliferation activity of mouse macrophage RAW 264.7 cells were detected. There were no significant differences in cell proliferation between the control group and the RAW 264.7 cells subjected to the different polar fractions of O.vulgaris (25, 50, 100, and 200 μg/ml) (Figure 4A; p > 0.05). When the concentration of the different polar fractions of O.vulgaris was greater than 200 μg/ml, the viability of the ethyl acetate- and n-butanol extract-treated cells decreased significantly (p < 0.01). The experimental results showed that the different polar fractions of O.vulgaris had no effect on cell proliferation and cytotoxicity at 25, 50, 100, and 200 μg/ml concentrations; thus, they were selected for subsequent experiments.
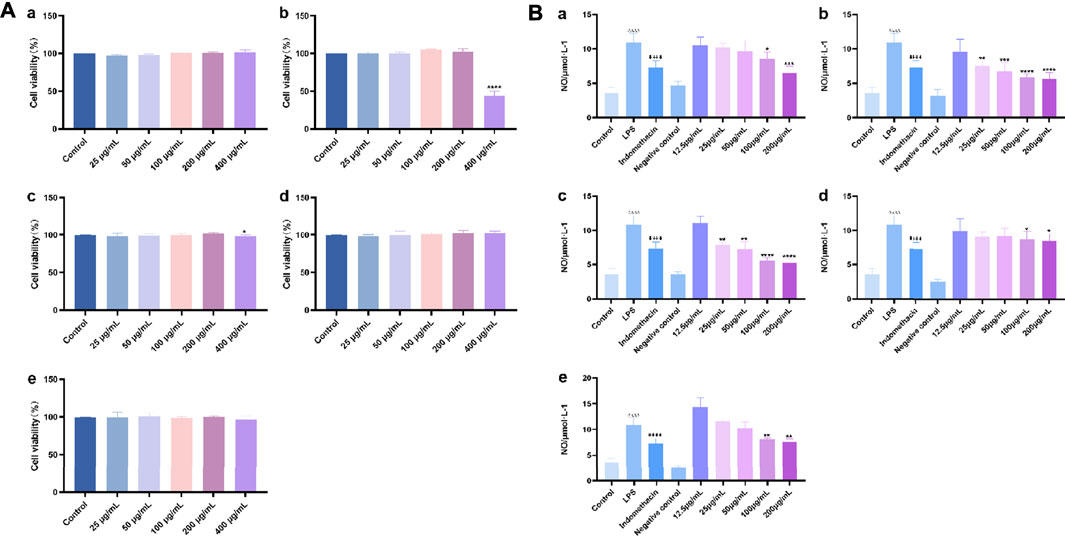
FIGURE 4. (A) Effect of the four O.vulgaris extracts on viability of RAW 264.7. (Aa) alcohol extract. (Ab) ethyl acetate extract. (Ac) n-butanol extract. (Ad) water extract. (Ae) petroleum ether extract. Data are presented as means ± SD, n = 5. * means p < 0.05, ** means p < 0.01, *** means p < 0.001, **** means p < 0.0001 compared with control group; (B) Effects of Different crude extracts of O.vulgaris on NO secretion in RAW 264.7 cells induced by LPS. (Ba) alcohol extract. (Bb) ethyl acetate extract. (Bc) n-butanol extract. (Bd) water extract. (Be) petroleum ether extract. Data are presented as means ± SD, n = 5. * means p < 0.05, ** means p < 0.01, *** means p < 0.001, **** means p < 0.0001 compared with LPS group. #### means p < 0.0001 compared with LPS group. △△△△ means p < 0.0001 compared with control group.
Cell Viability Assay and Protective Effects of the Extracts on LPS-Treated RAW 264.7 Cells
Analysis of the protective effects of the different crude extracts of O.vulgaris on LPS-induced inflammation in RAW 264.7 cells showed that the release of NO in the LPS model group was significantly higher than that in the control group (p < 0.0001). Compared with the LPS model group, the ethyl acetate (Figure 4B-b) and n-butanol fractions (Figure 4B-c) at concentrations of 25, 50, 100, and 200 μg/ml significantly inhibited the release of NO (p < 0.05) in a concentration-dependent manner. This indicated that the ethyl acetate and n-butanol fractions could improve the inflammatory response in LPS-induced mouse macrophage RAW 264.7 cells in the experimental concentration range. IC50 values were calculated according to the concentrations of the ethyl acetate and n-butanol fractions of O.vulgaris. The n-butanol fraction (135.9 μg/ml) showed a higher anti-inflammatory effect than the ethyl acetate fraction (166.8 μg/ml). The alcohol, water, and petroleum ether fractions (Figures 4B-a,d,e) had an inhibitory effect on NO secretion by RAW 264.7 cells stimulated with LPS at 100 and 200 μg/ml concentrations that showed a statistically significant difference to that on NO secretion in the LPS model group (p < 0.05). There was no statistically significant inhibitory effect at other concentrations (p > 0.05).
Chemical Isolation of Pure Compounds
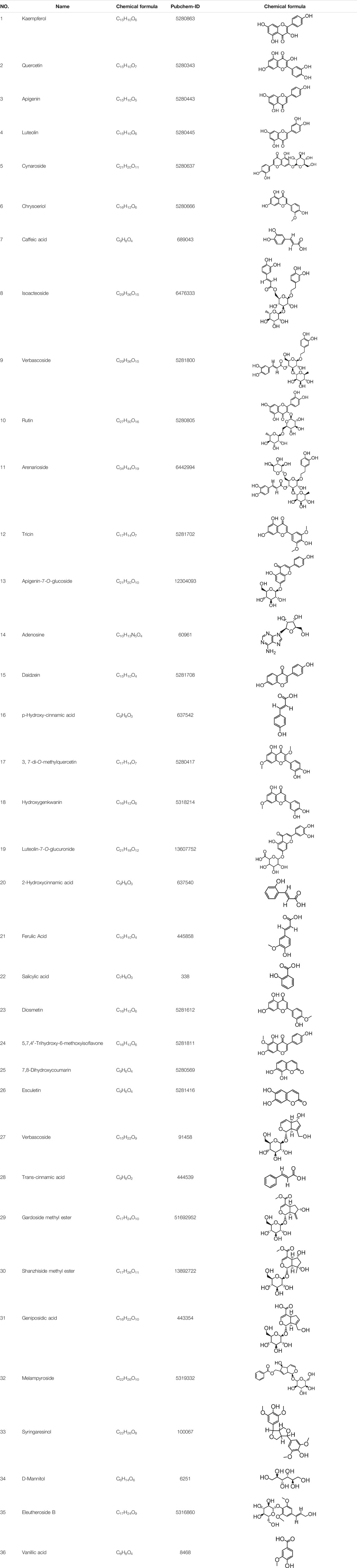
The structures of the following 17 compounds isolated from O.vulgaris were identified by (1H-NMR, 13C-NMR and ESI-MS) techniques: D-mannitol (compound 1) (Wang 2015), geniposidic acid (compound 2) (Ge et al., 2017), salidroside (compound 3) (Qu et al., 2020), shanzhiside methyl ester (compound 4) (Zhang et al., 2019a), eleutheroside B (compound 5) (Meng et al., 2020), geniposide (compound 6) (Li et al., 2016), 7,8-dihydroxycoumarin (compound 7) (Zhou et al., 2021), gardoside methyl ester (compound 8) (Cai et al., 2013), arenarioside (compound 9) (Pan 2011), verbascoside (compound 10) (Zhang et al., 2019a), isoacteoside (compound 11) (Zhang et al., 2019a), vanillic acid (compound 12) (Wang et al., 2020c), p-hydroxy-cinnamic acid (compound 13) (Shang et al., 2019), melampyroside (compound 14) (Vogl et al., 2013), syringaresinol (compound 15) (Xia et al., 2021), tricin (compound 16) (Zhou et al., 2021), and diosmetin (compound 17) (Yang et al., 2020). The structures of the 17 monomers are shown in Figure 5A. Of them, compounds 1–9 and 12–17 were isolated from O.vulgaris for the first time.
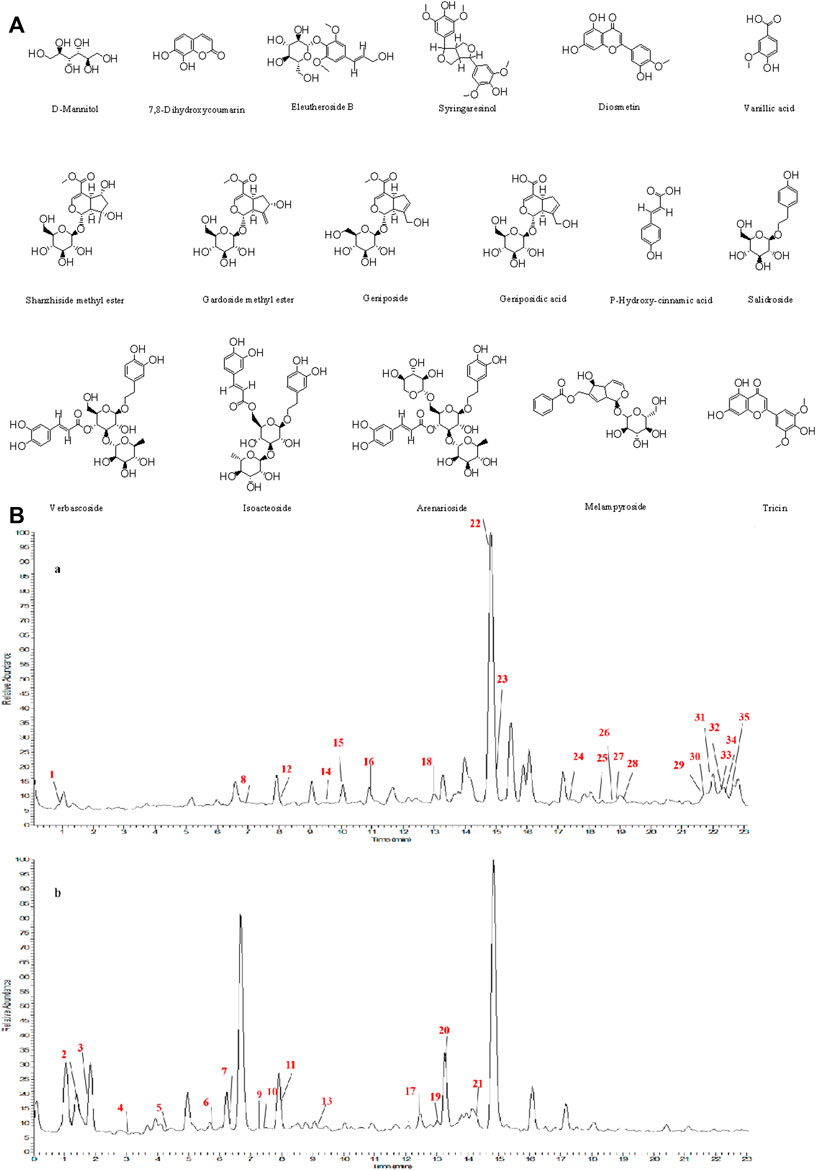
FIGURE 5. (A) The chemical structures of 17 monomeric compounds from O.vulgaris. (B) The total ion chromatograms (TIC) of ethyl acetate extract (Ba) and n-butanol extract (Bb) form the O.vulgaris in Positive mode.
UPLC-MS Analysis
Using UPLC–MS, 35 compounds were identified from the n-butanol and ethyl acetate fractions, of which 16 were identified by the reference substance. The chemical compounds of the n-butanol and ethyl acetate fractions were preliminarily determined and were mainly divided into four categories: phenylethanoid glycosides (verbascoside, isoacteoside, arenarioside, salidroside), flavonoids (kaempferol, cynaroside, luteolin, luteolin-7-O-glucuronide, apigenin, apigenin-7-O-glucoside, tricin, chrysoeriol, quercetin, diosmetin, hydroxygenkwanin), iridoids (melampyroside, shanzhiside methyl ester, aucubin, geniposide, geniposidic acid, shanzhiside methyl ester), phenolic acids (caffeic acid, trans-cinnamic acid, 2-hydroxycinnamic acid, salicylic acid, ferulic acid, 7,8-dihydroxycoumarin), and others (adenosine, syringaresinol, D-Mannitol, esculetin). The total ion chromatograms of the ethyl acetate and n-butanol extracts of O. vulgaris, both in the positive ion mode, are presented in Figure 5B. The retention time, chemical formula, measured mass, and theoretical mass of the 35 identified compounds are presented in Table 1.
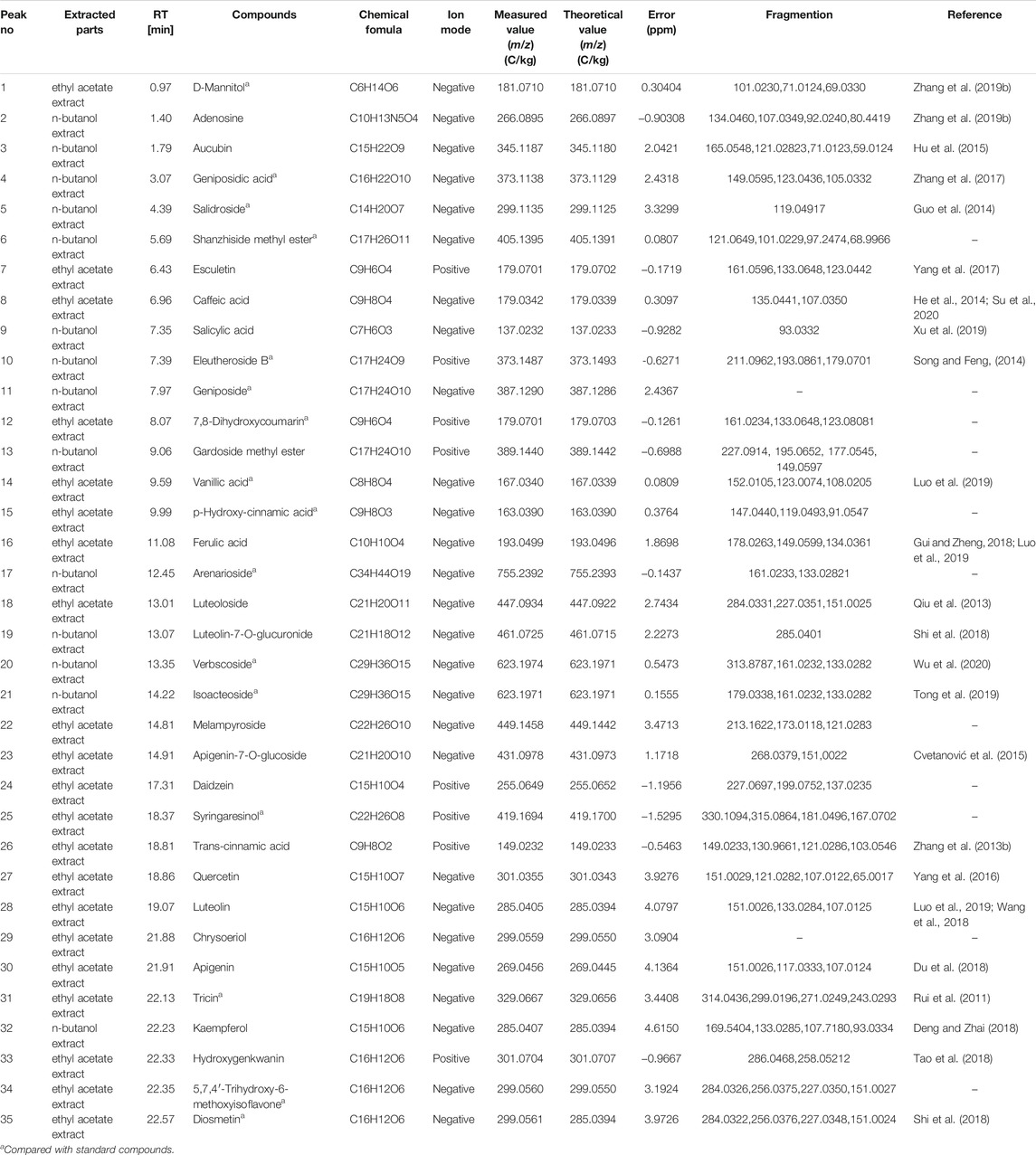
TABLE 1. Identification of major chemical compounds in n-butanol extract and ethyl acetate extract form the O.vulgaris.
The characteristics of the representative compounds in the ethyl acetate and n-butanol fractions of the O.vulgaris were as follows: given its molecular ion peak of m/z 449.1458 in negative ion mode, the molecular formula of peak 22 (melampyroside) was determined to be C22H26O10. Accordingly, a second-order spectrum was found, and fragment ion peaks at m/z 213.1622, 173.0118, and 121.0283 were recorded. Caffeic acid (peak 8) was detected in the ethyl acetate fraction of the herb, and its fragment ion peaks were at m/z 135.0441 and 107.0350. Peak 20 of n-butanol showed a molecular ion peak at m/z 623.1974, which was identified as that of verbascoside. There were fragment ions peaks at m/z 313.8787, 161.0232, and 133.0282 in the secondary spectrum, which was consistent with the fragmentation mode of the reference substance and that in previous reports. The molecular ion peak at m/z 623.1971 of peak 21, which was speculated to be that of the isomer of verbascoside, also showed a similar fragmentation mode. On comparison with the fragmentation mode of the reference substance, peak 21 was identified as that of isoacteoside. Peak 27 at m/z 301.0355 in the ethyl acetate fraction was identified as that of quercetin. The fragment ions with peaks at m/z 151.0029, 121.0282, 107.0122, and 65.0017 were found in the secondary spectrum, which was consistent with the fragmentation mode of the reference substance.
Network Pharmacology
Chemical Composition of O. vulgaris
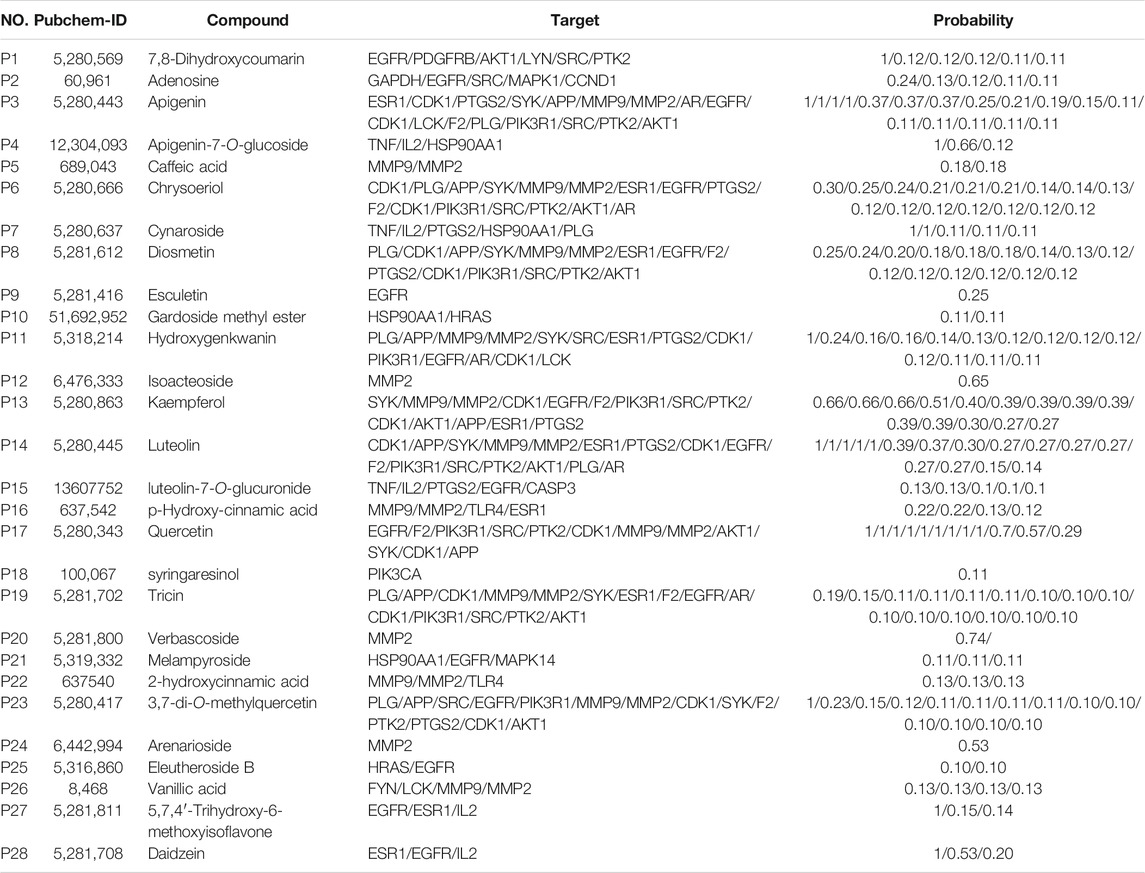
The known and predicted targets of the 36 compounds, after eliminating duplicates, were examined (Table 2). In this study, we only included results with probability >0.1 as the prediction target, and there were 294 targets in total.
Targets Relevant to O. vulgaris Treatment of RA
A total of 5,387 RA-related genes were compared with the target genes of 36 compounds to determine the potential therapeutic effect of each compound on RA target genes. The potential processing capacity of O. vulgaris to affect RA target genes was comprehensively obtained, and a total of 198 target genes were reported (Figure 6A).
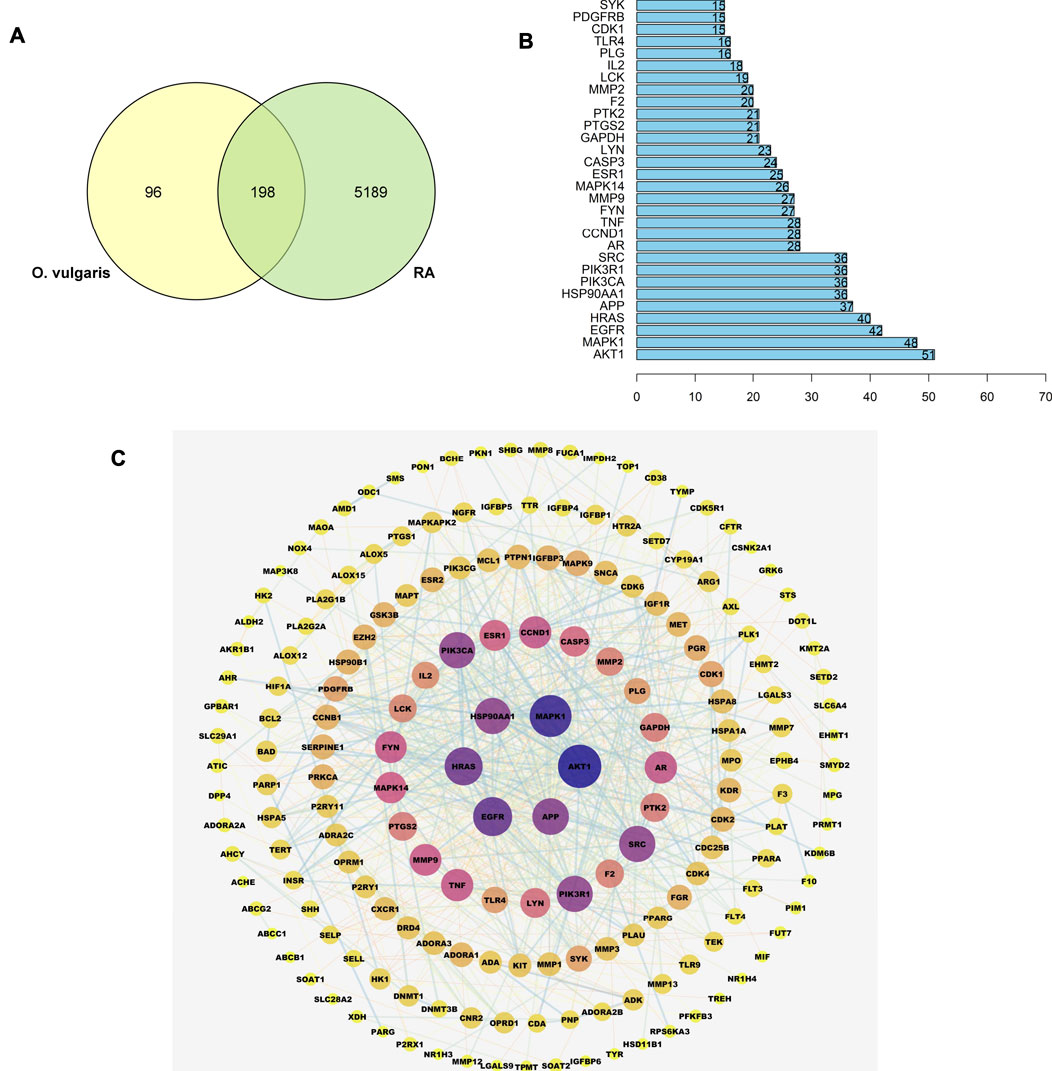
FIGURE 6. (A) Overlapping genes between RA-related genes and 36 compounds of O. vulgaris related genes; (B) The core gene for O.vulgaris treat RA; (C) PPI network diagram.
Construction and Analysis of the PPI Network of the Target Protein
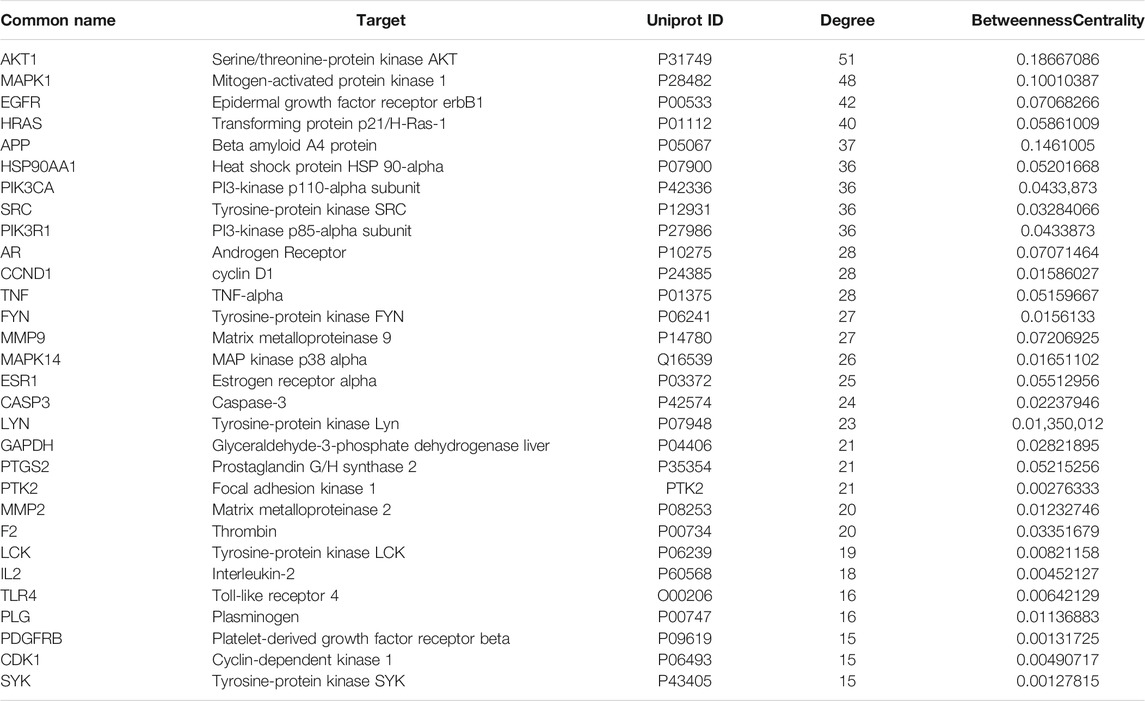
A PPI network of the candidate target protein of O. vulgaris was constructed. A visual PPI network diagram was generated to evaluate the topology parameters of the network nodes. A total of 198 nodes and 552 edges were obtained from these interactions. Nodes that were larger and darker in blue were those with higher degrees of freedom (Figure 6C). In PPI networks, nodes with a higher degree may play a more important role; thus, the top 30 proteins here may be key molecular targets for the treatment of RA. Further screening showed that 28 compounds were linked to the top 30 proteins, of which 14 were flavonoids, two were iridoids, three were phenylethanolglycosides, six were phenolic acids, and three were other compounds (Table 3). Akt1, MAPK1, EGFR, and HRAS were found to be associated with 51, 48, 42, and 40 proteins, respectively (Figure 6B; Table 4).
GO Analysis of Target Proteins
To verify the biological characteristics of 198 targets of O. vulgaris, GO enrichment analysis was performed on the presumed targets to clarify the related biological processes (Figure 7; p < 0.01). The top 10 significantly enriched terms in the BP (Biological Process), MF (Molecular Function), and CC (Cellular compound) categories were selected. Among them, BP (2,175 records), MF (167 records), and CC (92 records) accounted for 89.36, 6.86, and 3.78%, respectively. In the BP category, the target proteins were associated with cellular response to chemical stress (GO:0062197), response to oxidative stress (GO:0006979), and rhythmic processes (GO:0048511). In the MF category, the target proteins were associated with protein tyrosine kinase activity (GO:0004713), insulin-like growth factor I binding (GO:0031994), and phosphatase binding (GO:0019902). In the CC category, the target proteins were associated with membrane rafts (GO:0045121), membrane microdomains (GO:0098857), and vesicle lumen (GO:0031983).
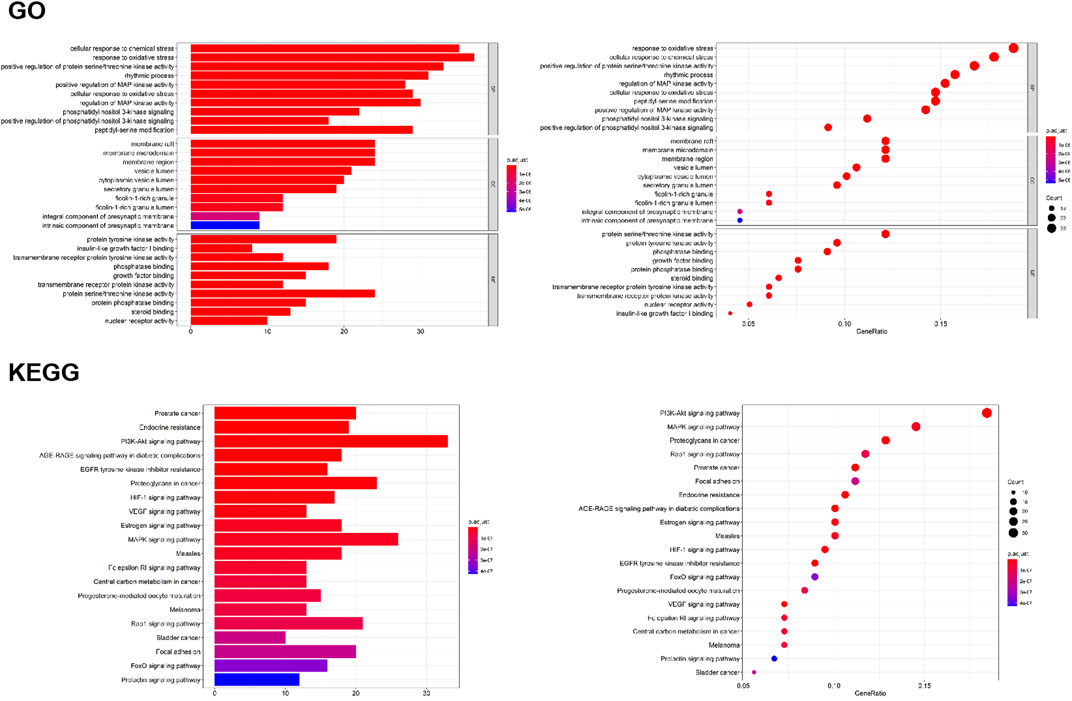
FIGURE 7. GO analyses of the 198 gene symbols associated with RA. [The x-axis showing the significant enrichment in the counts of these terms. The y-axis showing the categories of “biological process” in the GO of the target genes (p < 0.01)]; KEGG pathway enrichment analyses [The X-axis showing the counts of the target symbols in each pathway; the Y-axis showing the main pathways (p < 0.01)].
KEGG Classification of Target Proteins
To determine the potential pathway underlying the effects of O. vulgaris on RA treatment, KEGG pathway enrichment analysis was performed on 198 genes. Figure 7 shows the 20 most significant signaling pathways. According to these results, the most important pathways associated with the effects of O. vulgaris on RA treatment were the PI3K-Akt, HIF-1, VEGF, estrogen, MAPK, Fc epsilon RI, Rap1, FoxO, and Prolactir signaling pathways, suggesting that these pathways mediated the effect of O. vulgaris on RA.
Construction of Compound-Target and Target-Pathway Network
Based on the target prediction results and KEGG analysis results of the chemical compounds of O. vulgaris, the Cytoscape 3.7.1 software was used to construct the network of the “compound-target” and “target and pathway” of O. vulgaris in the treatment of RA for visual analysis. In this network, the orange nodes represented compounds, blue and pink nodes represented targets, green nodes represented pathways, and red and purple nodes represented O. vulgaris and RA, respectively. The potential target of a compound was linked by an edge (Figure 8A). In this study, 30 core genes and their corresponding 36 compounds were used to establish an “compound-target” map, and 20 pathways from KEGG enrichment results and their corresponding targets were used to establish a “target-pathway” map.
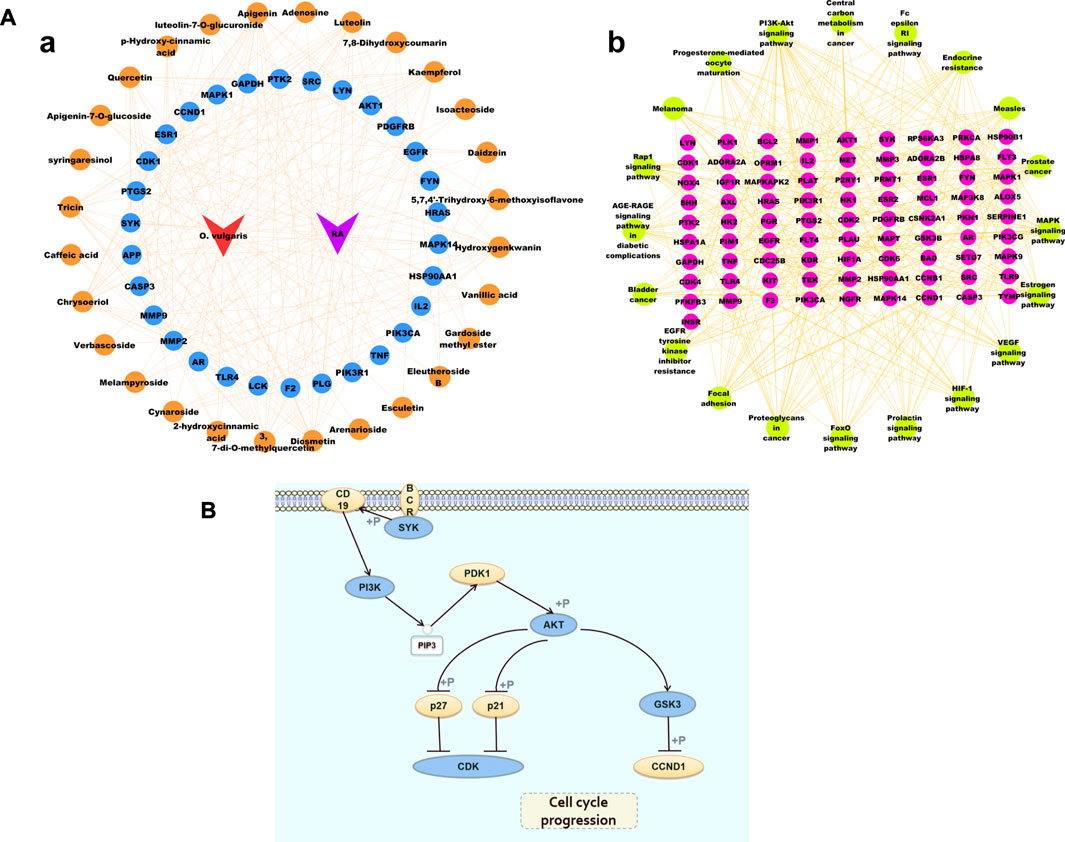
FIGURE 8. (A) Cytoscape Network diagram [(Aa). Compound-Target diagram; (Ab). Target-Pathway Diagram]; (B) PI3K-Akt signaling pathway.
Figure 8B shows the PI3K-Akt signaling pathway. The enrichment results showed that 17 common targets, namely, IGF1R, KDR, AKT1, PRKCA, PIK3R1, INSR, IL2, PTK2, CDK6, CDK2, GSK3B, FLT3, PIK3CG, SYK, KIT, MET, PKN1, and EGFR, were enriched in the PI3K-Akt signaling pathway.
Compound Analysis
Verbascoside, isoacteoside, melampyroside, and tricin were isolated from the ethyl acetate and n-butanol fractions and were representative of the effective compounds. According to the area normalization method, the relative peak areas of verbascoside, isoacteoside, tricin, and melampyroside in the n-butanol and ethyl acetate fractions of O. vulgaris were up to 47.97% and 21.89%, respectively. Tricin is a flavonoid that presents anti-inflammatory activity. It acts mainly by inhibiting cyclooxygenase (COX), TNF and prostaglandin (PG), IL, NO, and other inflammatory mediators. It has potential medicinal value for the treatment of RA (Zhan et al., 2018; Yin et al., 2020). Verbascoside, and isoacteoside are phenylethanolglycosides that have been shown to have significant anti-inflammatory activity and potential value in the development of new drugs for RA (Song et al., 2011). Melampyroside is an iridoid compound, and its relative peak areas in the ethyl acetate and n-butanol extracts reached 11.39% and 13.31%, respectively. Iridoids have been studied for their anti-inflammatory activity (Gui and Zheng, 2018). Taken together, the four selected compounds were representative and formed a basis for the verification experiment.
Molecular Docking
We selected the top 10 targets (AKT1, MAPK1, EGFR, HRAS, APP, HSP90AA1, PIK3CA, SRC, PIK3R1, and AR) in the PPI network and four compounds (verbascoside, isoacteoside, melampyroside, and tricin) for analysis of molecular docking. The docking result is depicted as a heat map display in Figure 9A. The docking visualization results are shown in Figure 9B. The results show that the binding energy of each compound to protein was less than −5 kcal/mol, indicating that each compound could bind well to protein. Of them, the binding energies of isoacteoside and tricin to SRC were −9.2 and −8.6 kcal/mol, respectively. The binding energies of verbascoside and melampyroside were −9.6 and −8.5 kcal/mol, respectively.

FIGURE 9. (A) Heat map of binding energy between the compounds of O. vulgaris and core targets molecule docking. (B) Docking modl diagram of compound and key target molecule.
Anti-Inflammatory Assay
Protective Effects of Target Compounds on LPS-Treated RAW 264.7 Cells
The effects of different target compounds (melampyroside, isoacteoside, verbascoside, and tricin) on the proliferation activity of mouse macrophage RAW 264.7 cells were examined. The results showed that there was no significant difference between the proliferation of control cells and that of RAW 264.7 cells upon treatment with different target compounds of O. vulgaris at 6.25, 12.5, 25, 50, and 100 μM (p > 0.05). When the concentrations of melampyroside and tricin were more than 100 μM, cell viability decreased significantly (p < 0.0001). The different target compounds of O. vulgaris had no effect on cell proliferation and cytotoxicity at 25, 50, and 100 μM concentrations; therefore, the 25, 50, and 100 μM concentrations were selected for subsequent experiments (Figure 10A).
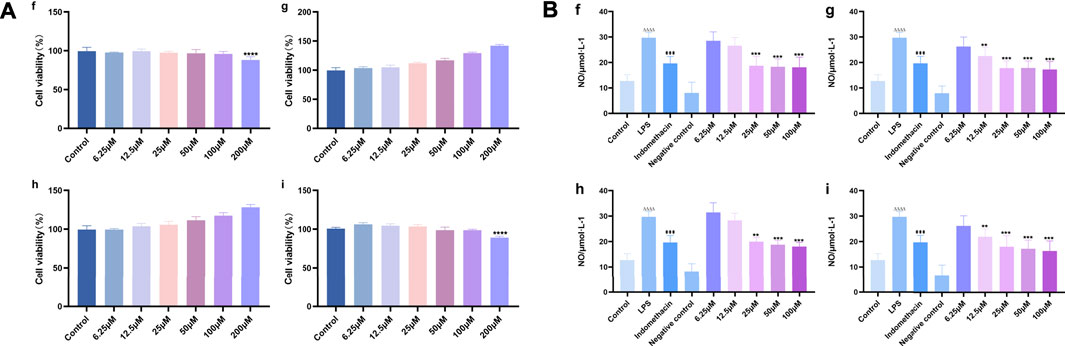
FIGURE 10. (A) Effect of target compounds of O. vulgaris extracts on viability of RAW 264.7 cell. (Af) melampyroside. (Ag) verbascoside. (Ah) isoacteoside. (Ai) tricin. Data are presented as means ± SD, n = 5. **** means p < 0.0001 compared with control group; (B) Effects of different target compounds of O.vulgaris on NO secretion in RAW 264.7 cells induced by LPS. (Bf) melampyroside. (Bg) verbascoside. (Bh) isoacteoside. (Bi) tricin. Data are presented as means ± SD, n = 5. * means p < 0.05, ** means p < 0.01, *** means p < 0.001, **** means p < 0.0001 compared with LPS group. #### means p < 0.0001 compared with LPS group. △△△△ means p < 0.0001 compared with control group.
Cell Viability Assay and the Protective Effects of Target Compounds on LPS-Treated RAW 264.7 Cells
NO release in the LPS model group was significantly higher than that in the normal control group (Figure 9B; p < 0.0001). The effect of different target compounds on NO release was not significant in the negative control group, and there was no significant difference compared with that in the normal control group (p > 0.05). Compared with that in the LPS model group, treatment with 6.25, 12.5, 25, 50, and 100 μM of verbascoside (Figure 10B-g), and tricin (Figure 10B-i) significantly inhibited the release of NO (p < 0.05) in a concentration-dependent manner. Melampyroside (Figure 10B-f) and isoacteoside (Figure 10B-h) treatment significantly inhibited the release of NO at concentrations of 25, 50, and 100 μM (p < 0.05). The IC50 values were calculated according to the concentrations of the different target compounds of O. vulgaris. The IC50 values were as follows: melampyroside, 129.7 μM; verbascoside, 129.9 μM; isoacteoside, 114.5 μM; and tricin, 101.6. Thus, the anti-inflammatory effect of the four target compounds was in following order: tricin > isoacteoside > verbascoside > melampyroside.
Enzyme-Linked Immunosorbent Assay
Compared with those in the control group, the levels of TNF-α, IL-6, and IL-1β in the model group were significantly increased (Figure 11;p < 0.0001), indicating that LPS caused an inflammatory response in RAW 264.7 cells. Compared with that in the model group, TNF-α content decreased significantly after treatment with different concentrations (25, 50, and 100 μM) of melampyroside, verbascoside, isoacteoside, and different concentrations (50 and 100 μM) of tricin. A concentration dependence was observed in the effective concentration analysis (p < 0.05). Compared with that in the model group, IL-1β content decreased significantly after treatment with different concentrations (25, 50, and 100 μM) of verbascoside, and different concentrations (50 and 100 μM) of tricin, isocalycoside, and melampyroside. A certain concentration dependence was observed (p < 0.05). Compared with that in the model group, IL-6 content decreased significantly after treatment with different concentrations (25, 50, and 100 μM) of melampyroside, verbascoside, and tricin, and different concentrations (50 and 100 μM) of isoacteoside. A concentration dependence was observed (p < 0.05). The results showed that melampyroside, verbascoside, isoacteoside, and tricin could downregulate the levels of inflammatory factors and exert anti-inflammatory effects. There was no significant difference seen between the verbascoside treated groups and the positive control group (indomethacin) in IL-1β content at 100 μM (p > 0.05). There was no significant difference in IL-6 content between the verbascoside, and tricin groups and the positive control group (indomethacin) at 100 μM (p > 0.05).
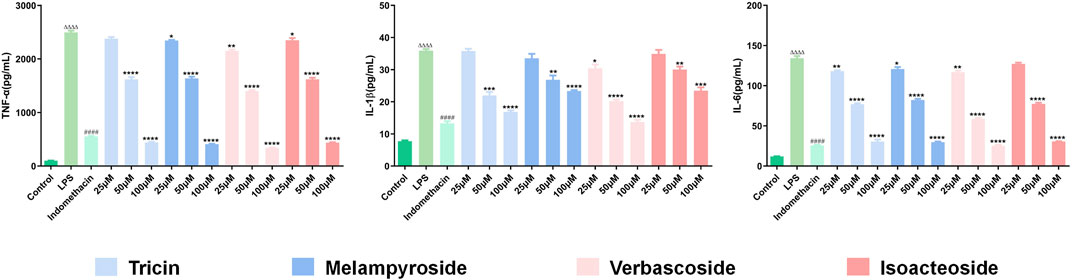
FIGURE 11. Effects of Different target compounds from O. vulgaris on TNF-α, IL-1β and IL-6 Content in RAW 264.7 Cells Induced by LPS. Data are presented as means ± SD, n = 5. * means p < 0.05, ** means p < 0.01, *** means p < 0.001, **** means p < 0.0001 compared with LPS group. #### means p < 0.0001 compared with LPS group. △△△△ means p < 0.0001 compared with control group.
Discussion
Based on the symptoms of RA and the pharmacodynamic basis of O. vulgaris, although O. vulgaris has enormous therapeutic potential, its full chemical characterization remains incomplete. Therefore, the purpose of this study was to evaluate the biological activity of O. vulgaris and analyze its chemical compounds to lay a foundation for the identification of new drugs.
In this study, anti-inflammatory activity was determined in terms of the NO content of RAW 264.7 mouse macrophages induced by LPS. The results showed that the ethyl acetate and n-butanol extracts exhibited better activity. We isolated 17 compounds from the n-butanol and ethyl acetate extracts of O. vulgaris with compounds 1–9 and 12–17 being isolated from O. vulgaris for the first time. The UPLC–Q-Exactive assay was used to identify 35 compounds from the ethyl acetate and n-butanol extracts of O. vulgaris.
Network pharmacological results showed that 36 compounds, including flavonoids, iridoids, phenolic acids, and phenylethanolglycosides, were directly related to the therapeutic effect of O. vulgaris on RA. In this study, the PI3K-Akt pathway was found to be particularly important for the treatment of RA by O. vulgaris. The PI3K-Akt signaling pathway is closely related to the occurrence and development of RA and plays an important role in the regulation of differentiation and formation of osteoblasts (Wang et al., 2019). Overproliferation of synovial cells and RA can be reversed via the use of P13K-Akt signaling inhibitors, or by the upregulation of endogenous negative regulatory proteins in the signaling pathway. This inhibits glycogen synthase kinase 3 (GSK3) activity and accelerates cell apoptosis. In RA, the earliest phenomenon is the formation of new blood vessels, which can develop into a pannus in severe cases. Vascular endothelial growth factors (VEGFs) are an important class of signaling proteins and ligands secreted by cells to stimulate vasculogenesis and angiogenesis (Da and Li, 2012). They are also as important inducers and direct pro-inflammatory factors. VEGF proteins play a vital role in activating angiogenesis in RA through the upregulation of the VEGF pathway (Yi et al., 2016; Meloa et al., 2020). Cheng et al. (2011) used the immunohistochemical SP method to detect VEGF protein expression in the synovial tissues of the knee joints of 27 patients with active RA. The results showed that the expression of VEGF in the synovial tissues of controls and patients with osteoarthritis was significantly lower than that in patients with RA.
According to the component-target interaction analysis, ten targets with higher degrees on the PPI network (AKT1, MAPK1, EGFR, HRAS, APP, HSP90AA1, PIK3CA, SRC, PIK3R1, and AR) were identified. The docking scores of all compound-target pairs were less than −5 kcal/mol, indicating that each of the four selected compounds (verbascoside, isoacteoside, melampyroside, and tricin) had good binding affinity to all 10 targets. The binding energies of isoacteoside, tricin to SRC were −9.2, −9.9, and −8.6 kcal/mol, respectively. The binding energies of verbascoside and melampyroside were −9.6 and −8.5 kcal/mol, respectively. The molecular docking simulation strengthened all the above investigations to some extent.
As a major pro-inflammatory factor in the pathogenesis of RA, TNF-α is considered the leading trigger for inflammation and joint destruction. It can stimulate the synovium, fibroblasts, osteoclasts, and other cells to produce destructive substances called matrix metalloproteinases, which can damage cartilage and gradually destroy bone tissue in patients with RA. Therefore, TNF-α level can be used as an indicator of inflammatory activity in RA (Wang et al., 2016). IL-6 is produced by synovial cells, and its abnormal expression and dysregulation are typical characteristics of RA [39]. IL-6 can enhance the effects of IL-1 and TNF-α and is considered to be an amplifying factor of some biological effects of TNF-α (Shi et al., 2013). At non-toxic doses, the four target compounds in O. vulgaris significantly reduced the secretion of TNF-α, IL-1β, IL-6, and NO, suggesting that O. vulgaris is a potential therapeutic agent for RA. The anti-inflammatory activity of O. vulgaris can be attributed to its bioactive compounds. Phenylethanolglycosides, flavonoids, and iridoids have a wide range of pharmacological activities, including anti-inflammatory activityies (Hanfer et al., 2017; Spagnuolo et al., 2018; Zhang et al., 2018). Verbascoside has shown anti-inflammatory activities, such as reducing the presence of pro-inflammatory factors in intestinal inflammation (Danae et al., 2013). As an isomeride of verbascoside, the similarities and differences in the anti-inflammatory activity of isoacteoside and the underlying mechanism of action require further exploration. Melampyroside is an iridoid terpene compound, and the relative peak area of the ethyl acetate and n-butanol fractions of O. vulgaris is relatively large. Therefore, pharmacological studies on O. vulgaris cannot be ignored. Tricin is a flavonoid, and its anti-inflammatory properties have been studied (Zhan et al., 2018; Yin et al., 2020); it is noteworthy that tricin has shown outstanding anti-inflammatory activity in in vitro experiments.
Based on studies of previous chemical substances, we used bioinformatics to predict the targets and potential action pathways of O. vulgaris in the treatment of RA and detected its target compounds from the levels of inflammatory factors in vitro. The results of network analysis and prediction showed that the PI3K-Akt pathway may play an important role in O. vulgaris-mediated treatment of RA. Therefore, the development of anti-inflammatory drugs using O. vulgaris has considerable advantages. However, bioinformatics methods still have some limitations, for example, it is found that it is too one-sided to explore the efficacy of medicinal plants only from the perspective of network pharmacology. It is necessary to consider the relative content of compounds in medicinal plants, so as to give better play to the pharmacological role of medicinal plants. In addition, the binding energy of compounds in molecular docking experiments is not necessarily at the same level as their therapeutic potential in vivo, and further systematic molecular biology experiments are needed to verify the accurate mechanisms.
In summary, the results of this study support the potential anti-inflammatory activity of O. vulgaris, and combined with computer pharmacology, it is speculated that it may have the activity of treating RA. In the development of Chinese and Mongolian medicine, O. vulgaris could be a potential source of novel drugs for the treatment of RA. Our study also provides a novel paradigm to identify the potential mechanisms of pharmacological effects derived from a natural compound.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
ML conceived the research ideas. XW advised on data collection. MJ and CW designed the experiments in detail and provided valuable guidance on data analysis. ML and TY provided important insights and suggestions for this research. XM aided in data consolidation. MJ and CW performed the majority of the data processing and wrote the manuscript. All authors significantly contributed to the manuscript and have read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 81874336), China Agriculture Research System (Grant No. CARS-21), 2019 Medical Service and Security Capacity Enhancement Subsidy Project (Grant No. Finance Society (2019) 39).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, P., Xin, S. S., and Dong, Y. (2015). The Study Progress of Mongolian Medicine Treatment of Rheumatoid Arthritis. J. Inner Mongolia Med. Univ. (s1), 12–15. doi:10.16343/j.cnki.issn.2095-512x.2015.s1.003
Burley, S. K., Berman, H. M., Kleywegt, G. J., Markley, J. L., Nakamura, H., and Velankar, S. (2017). Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. 1607, 627–641. doi:10.1007/978-1-4939-7000-1_26
Cai, C. J., Zhang, Z. L., Zuo, Y. M., Zhu, Y. Y., Luo, G. M., and Zhang, J. (2013). Studies on the Chemical Components of Iridoids of Gardenia Jasminoides Ellis. Lishizhen Med. Mater. Med. Res. 24, 342–343. doi:10.3969/j.issn.1008-0805.2013.02.041
Carrillo-Ocampo, D., Bazaldúa-Gómez, S., Bonilla-Barbosa, J. B. B., Aburto-Amar, R., and Rodríguez-López, V. (2013). Anti-inflammatory Activity of Iridoids and Verbascoside Isolated from Castilleja Tenuiflora. Molecules 18, 12109–12118. doi:10.3390/molecules181012109
Cheng, Q., Song, W. G., Wang, N., Zhang, Y. X., and Zhang, Y. P. (2011). Expression and Clinical Significance of VEGF in Rheumatoid Arthritis Synovium. Chin. Mod. Med. 18, 70–71. doi:10.3969/j.issn.1674-4721.2011.25.038
Cicek, M., Unsal, V., Doganer, A., and Demir, M. (2021). Investigation of Oxidant/antioxidant and Anti-inflammatory Effects of Apigenin on Apoptosis in Sepsis-Induced Rat Lung. J. Biochem. Mol. Tox. e22743. doi:10.1002/jbt.22743
Cvetanović, A., Švarc-Gajić, J., Zeković, Z., Savić, S., Vulić, J., Mašković, P., et al. (2015). Comparative Analysis of Antioxidant, Antimicrobiological and Cytotoxic Activities of Native and Fermented Chamomile Ligulate Flower Extracts. Planta 242, 721–732. doi:10.1007/s00425-015-2308-2
Da, G. L., and Li, H. B. (2012). Vascular Endothelial Growth Factor Role in the Angiogenesis of Rheumatoid Arthritis and the Related Research Progress. Med. Recapit. 18, 644–648. doi:10.3969/j.issn.1006-2084.2012.05.002
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 47, W357. doi:10.1093/nar/gkz382
Deng, J. L., Xu, Y. H., and Wang, G. (2019). Identification of Potential Crucial Genes and Key Pathways in Breast Cancer Using Bioinformatic Analysis. Front. Genet. 10, 695. doi:10.3389/fgene.2019.00695
Deng, Y. F., and Zhai, X. J. (2018). Simultaneous Determination of Astragulin and Kaempferol in Bairui Granule by HPLC-MS/MS. China Pharm. 21, 524–526.
Du, G., Fu, L., Jia, J., Pang, X., Yu, H., Zhang, Y., et al. (2018). Validated UPLC-MS/MS Method for Quantification of Seven Compounds in Rat Plasma and Tissues: Application to Pharmacokinetic and Tissue Distribution Studies in Rats after Oral Administration of Extract of Eclipta Prostrata L. Biomed. Chromatogr. 32, e4191. doi:10.1002/bmc.4191
Ferreira de Melo, I. M., Martins Ferreira, C. G., Lima da Silva Souza, E. H., Almeida, L. L., Bezerra de Sá, F., Cavalcanti Lapa Neto, C. J., et al. (2020). Melatonin Regulates the Expression of Inflammatory Cytokines, VEGF and Apoptosis in Diabetic Retinopathy in Rats. Chemico-Biological Interactions 327, 109183. doi:10.1016/j.cbi.2020.109183
Ge, W., Li, H. B., Yu, Y., Fang, H., Meng, Z. Q., Huang, W. Z., et al. (2017). Research on Chemical Constituents from Reduning Injection (IV). Chin. Tradit. Herb. Drugs 48, 3042–3050. doi:10.7501/j.issn.0253-2670.2017.15.005
Gong, X., Wang, J., Zhang, M., Wang, P., Wang, C., Shi, R., et al. (2020). Bioactivity, Compounds Isolated, Chemical Qualitative, and Quantitative Analysis of Cymbaria Daurica Extracts. Front. Pharmacol. 11, 48. doi:10.3389/fphar.2020.00048
Gui, Q., and Zheng, J. (2018). Simultaneous Determination of Eight Components in Angelica Sinensis Based on UHPLC-ESI-MS/MS Method for Quality Evaluation. Biomed. Chromatogr. 33, e4326. doi:10.1002/bmc.4326
Guo, N., Ding, W., Wang, Y., Hu, Z., Wang, Z., and Wang, Y. (2014). An LC-MS/MS Method for the Determination of Salidroside and its Metabolite P-Tyrosol in Rat Liver Tissues. Pharm. Biol. 52, 637–645. doi:10.3109/13880209.2013.863946
Guo, X., Ji, J., Feng, Z., Hou, X., Luo, Y., and Mei, Z. (2020). A Network Pharmacology Approach to Explore the Potential Targets Underlying the Effect of Sinomenine on Rheumatoid Arthritis. Int. Immunopharmacol 80, 106201. doi:10.1016/j.intimp.2020.106201
Hanfer, M., Cheriet, T., Ameddah, S., Mancini, I., Seghiri, R., Menad, A., et al. (2017). Iridoids and Anti-inflammatory Properties of N-Butanol Extract of Linaria Tingitana Boiss. & Reut. Nat. Prod. Res. 31, 2008–2015. doi:10.1080/14786419.2016.1272111
Hao, M., Cheng, T., Wang, Y., and Bryant, H. S. (2013). Web Search and Data Mining of Natural Products and Their Bioactivities in PubChem. Sci. China Chem. 56, 1424–1435. doi:10.1007/s11426-013-4910-0
He, F., Zhang, J., Mou, J. L., Zheng, L., Lan, Y. Y., and Huang, Y. (2014). Simultaneous Determination and Pharmacokinetics of Albiflorin, Caffeic Acid and Chlorogenic Acid in Rat Plasma by UPLC-MS. Lishizhen Med. Mater. Med. Res. 25, 1803–1806.
Hu, F., An, J., Li, W., Zhang, Z., Chen, W., Wang, C., et al. (2015). UPLC-MS/MS Determination and Gender-Related Pharmacokinetic Study of Five Active Ingredients in Rat Plasma after Oral Administration of Eucommia Cortex Extract. J. Ethnopharmacol. 169, 145–155. doi:10.1016/j.jep.2015.04.007
Jiang, Y., Zhong, M., Long, F., Yang, R., Zhang, Y., and Liu, T. (2019). Network Pharmacology-Based Prediction of Active Ingredients and Mechanisms of Lamiophlomis Rotata (Benth.) Kudo against Rheumatoid Arthritis. Front. Pharmacol. 10, 1435. doi:10.3389/fphar.2019.01435
Li, J. B., Zhou, Q., Chen, F. F., Hua, A. L., Wang, S. Z., and Wu, M. T. (2019a). Pharmacokinetics and Bioavailability of Kaempferol in Rat Plasma by UPLC-MS/MS(Article). Lat. Am. J. Pharm. 38, 80–84.
Li, L., and Gu, W. Y. (2020). Resveratrol-mediated Tenascin-c/TLR4 Signaling Pathway Regulating Inflammatory Reaction in RA Rats. West. J. Tradit. Chin. Med. 33, 20–23.
Li, T., Gao, D., Du, M., Cheng, X., and Mao, X. (2018). Casein Glycomacropeptide Hydrolysates Inhibit PGE2 Production and COX2 Expression in LPS-Stimulated RAW 264.7 Macrophage Cells via Akt Mediated NF-Κb and MAPK Pathways. Food Funct. 9, 2524–2532. doi:10.1039/c7fo01989k
Li, X., He, H. H., Lin, D., Xie, Y. Q., and Xiao, M. (2019b). Prediction of the Mechanism of Action of Paederia Scandens in the Treatment of Rheumatoid Arthritis Based on Network. Hainan Med. J. 30, 3133–3139. doi:10.3969/j.issn.1003-6350.2019.24.002
Li, Y., Wang, X. L., Song, J. L., and Qi, H. Y. (2016). Chemical Constituents from Leaves of Gardeniae Fructus. Chin. J. Exp. Tradit. Med. Formulae. 22, 68–70.
Liu, F., Liu, Y., Zhan, S. P., Lv, J., Yu, M. J., and Xia, P. Y. (2019). Mechanism of Tibetan Medicine Terminalia Chebula Retz in Rheumatoid Arthritis: a Study Based on Network Pharmacology. J. Third Mil. Med. Univ. 41, 2238–2245.
Liu, Z. D., Jiang, M., Tan, Y., He, X. J., Jia, D. M., Zhou, K., et al. (2015). Research on Network Pharmacology of Cold or Heat Herbal Formula Correspond with Cold or Heat Syndromes of Rheumatoid Arthritis. Chin. J. Tradit. Chin. Med. Pharm. 30, 3191–3195.
Luo, X. M., Su, M. F., Chang, X. Y., Wang, X. M., Li, Z. Z., Wang, W. H., et al. (2019). Qualitative and Quantitative Analysis of Main Chemical Constituents in Eucommia Ulmoides by LC-MS. Mod. Chin. Med. 21, 1029–1040. doi:10.13313/j.issn.1673-4890.20190129004
Mao, G. H., Zhang, L. Q., Qian, F., Chen, K. X., Xu, J. W., and Li, J. M. (2019). Research Progress on Anti-inflammatory Activity of Three Kinds of Iridoid Glycosides in Chinese Materia Medica. Chin. Tradit. Herb. Drugs 50, 228–236.
Meng, X. H., Shang, X. Y., and Yang, J. L. (2020). Phenolic Chemical Constituents of Zanthoxylum Schinifolium Pericarps and Their Anti-oxidative Effects. Chin. Tradit. Herb. Drugs 51, 2095–2101. doi:10.7501/j.issn.0253-2670.2020.08.015
Nigro, O., Tuzi, A., Tartaro, T., Giaquinto, A., Vallini, I., and Pinotti, G. (2020). Biological Effects of Verbascoside and its Anti-inflammatory Activity on Oral Mucositis: a Review of the Literature. Anticancer Drugs 31, 1–5. doi:10.1097/CAD.0000000000000818
Pan, Y. N. (2011). Study on Chemical Constituents and Biological Activities of Fresh Products of Cistanche Tubulosa. Shenyang: Shenyang Pharmaceutical University.
Qiu, F., Li, Z., He, L., and Wang, D. (2013). HPLC-ESI-MS/MS Analysis and Pharmacokinetics of Luteoloside, a Potential Anticarcinogenic Component Isolated from Lonicera japonica, in Beagle Dogs. Biomed. Chromatogr. 27, 311–317. doi:10.1002/bmc.2793
Qu, S. Y., Wei, X. D., Sai, M., Lan, X. Z., and Chen, M. (2020). Chemical Constituents from Rhodiola Crenulata. Chin. Tradit. Pat. Med. 42, 3199–3203.
Rui, W., Feng, Y. F., Liu, S. J., and Tan, Y. Z. (2011). Analysis of Flavonoids in Ranunculus Japonicus by UPLC I-IILIC/Q-TOF-MS. Asia-pacific Tradit. Med. 7, 18–21.
Shang, Z. M., Cheng, L., Liu, G. Y., Zhang, M. S., Li, X. F., and Xiao, S. J. (2019). Chemical Constituents of Dendrobium Bellatulum. Chin. Tradit. Herb. Drugs 50, 2036–2040. doi:10.7501/j.issn.0253-2670.2019.09.005
Shi, F., Pan, H., Lu, Y., and Ding, L. (2018). An HPLC-MS/MS Method for the Simultaneous Determination of Luteolin and its Major Metabolites in Rat Plasma and its Application to a Pharmacokinetic Study. J. Sep. Sci. 41, 3830–3839. doi:10.1002/jssc.201800585
Shi, L. P., He, Y., Xun, W., and Wei, Y. L. (2013). The Role of the Combination Therapy on the Levels of Pro-inflammatory Cytokines and Acute Phase Reactants in Rheumatoid Arthritis. Pract. J. Clin. Med. 10, 78–80.
Song, G. X., Ma, L. Y., Wei, F., and Ma, S. C. (2011). The Distribution of Mangiferin Benzyl Ethanol Research Progress and Pharmacological Activities. Asia-pacific Tradit. Med. 7, 169–171.
Song, Y., and Feng, X. S. (2014). UPLC-MS/MS Analysis of the Constituents in the Roots of A. Sessiliflorus and Comparison with A. Gracilistylus and A. Senticosus. Chin. J. Pharm. Anal. 34, 958–965.
Spagnuolo, C., Moccia, S., and Russo, G. L. (2018). Anti-inflammatory Effects of Flavonoids in Neurodegenerative Disorders. Eur. J. Med. Chem. 153, 105–115. doi:10.1016/j.ejmech.2017.09.001
Su, Y. P., Ye, X. L., Li, H., Xu, W., and Chu, K. D. (2020). Simultaneous Determination of Caffeic Acid,protocatechuic Acid and Chlorogenic Acid in Gnaphalium Affine by UPLC-MS/MS. J. Int. Pharm. Res. 47, 1001–1005. doi:10.13220/j.cnki.jipr.2020.11.017
Szklarczyk, D., Morris, J. H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., et al. (2017). The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 45, D362–D368. doi:10.1093/nar/gkw937
Tanchuk, V. Y., Tanin, V. O., Vovk, A. I., and Poda, G. (2016). A New, Improved Hybrid Scoring Function for Molecular Docking and Scoring Based on AutoDock and AutoDock Vina. Chem. Biol. Drug Des. 87, 618–625. doi:10.1111/cbdd.12697
Tao, Y., Su, D., Li, W., and Cai, B. (2018). Pharmacokinetic Comparisons of Six Components from Raw and Vinegar-Processed Daphne Genkwa Aqueous Extracts Following Oral Administration in Rats by Employing UHPLC-MS/MS Approaches. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 1079, 34–40. doi:10.1016/j.jchromb.2018.02.005
Tian, C., Liu, X., Chang, Y., Wang, R., Lv, T., Cui, C., et al. (2021). Investigation of the Anti-inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. South Afr. J. Bot. 137, 257–264. doi:10.1016/j.sajb.2020.10.022
Tong, C., Xu, J., Fu, Q., Long, R., Peng, S., and Shi, S. (2019). Rapid Extraction, Discrimination and Quantification of Thermally Unstable Isomeric Acteoside and Isoacteoside in Natural Products by Online Extraction-Quadrupole Time-Of-Flight Tandem Mass Spectrometry. Anal. Methods 11, 2148–2154. doi:10.1039/C8AY02584C
Vetal, S., Bodhankar, S. L., Mohan, V., and Thakurdesai, P. A. (2013). Anti-inflammatory and Anti-arthritic Activity of Type-A Procyanidine Polyphenols from Bark of Cinnamomum Zeylanicum in Rats. Food Sci. Hum. Wellness 2, 59–67. doi:10.1016/j.fshw.2013.03.003
Vogl, S., Atanasov, A. G., Binder, M., Bulusu, M., Zehl, M., Fakhrudin, N., et al. (2013). The Herbal Drug Melampyrum Pratense L. (Koch): Isolation and Identification of its Bioactive Compounds Targeting Mediators of Inflammation. Evid. Based Complement. Alternat Med. 2013, 395316. doi:10.1155/2013/395316
Wang, B., He, Y. W., Zhao, L., and Ji, W. P. (2020a). Study on Effect of Aconite Decoction on Anti-inflammation and Joints of Rats with Chronic Infectious Arthritis. Chin. Mod. Doct. 58, 40–44.
Wang, C. F., Xu, J. Z., Wang, S. Y., Wang, Q. H., Yang, B. Y., and Kuang, H. X. (2020c). Study on the Chemical Constituents of Antibacterial Part from Medicated Leaven Massa. Lishizhen Med. Mater. Med. Res. 31, 2350–2353.
Wang, H., Zhou, X. M., Wu, L. Y., Liu, G. J., Xu, W. D., Zhang, X. S., et al. (2020d). Aucubin Alleviates Oxidative Stress and Inflammation via Nrf2-Mediated Signaling Activity in Experimental Traumatic Brain Injury. J. Neuroinflammation 17, 188. doi:10.1186/s12974-020-01863-9
Wang, R. S., Liu, J., Tong, P., He, S. J., Wu, Y. W., Zuo, J. P., et al. (2016). Hydroxytriptolide (LLDT-8) Inhibits Inflammatory Cytokines and Promotes Apoptosis in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes by TNF-α Induced via Ras-MAPKs Signaling Pathway. Acta Univ. Tradit. Med. Sinensis Pharmacol. Shanghai. 30, 82–87.
Wang, X., Jiang, Z. T., Yan, G. J., Shao, Z. Y., Feng, X. Y., and Pan, J. H. (2018). Simultaneous Determination of 8 Components in Total Flavanones of Sedum Sarmentosum Bunge by UPLC-MS/MS. Chin. Pharm. 29, 1222–1226.
Wang, X. Y. (2015). Study on Chemical Constituents and Pharmacological Activity of Pholiota Adiposa. Jilin: Jilin Agricultural University.
Wang, Y., Zhou, Z., Han, M., Zhai, J., Han, N., Liu, Z., et al. (2020b). The Anti-inflammatory Components from the Effective Fraction of Syringae Folium (ESF) and its Mechanism Investigation Based on Network Pharmacology. Bioorg. Chem. 99, 103764. doi:10.1016/j.bioorg.2020.103764
Wang, Z., Linghu, K. G., Hu, Y., Zuo, H., Yi, H., Xiong, S. H., et al. (2019). Deciphering the Pharmacological Mechanisms of the Huayu-Qiangshen-Tongbi Formula through Integrating Network Pharmacology and In Vitro Pharmacological Investigation. Front. Pharmacol. 10, 1065. doi:10.3389/fphar.2019.01065
Wu, L., Du, S., Yang, F., Ni, Z., Chen, Z., Liu, X., et al. (2020). Simultaneous Determination of Nineteen Compounds of Dahuang Zhechong Pill in Rat Plasma by UHPLC-MS/MS and its Application in a Pharmacokinetic Study. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 1151, 122200. doi:10.1016/j.jchromb.2020.122200
Xia, Z., Zhang, H. X., Xu, T. Q., Chen, Y. M., and Zhou, G. X. (2021). Phenylpropanoids from Fruits of Xanthium Sibiricum. Chin. Pharm. J. 56, 13–22. doi:10.1080/14786419.2020.1806273
Xu, A. L., Chen, Z., Bi, X. L., Jiang, J. Y., Zhang, J. N., Li, S. M., et al. (2019). Simultaneous Determination of Five Components in Jasminum Elongatum by UHPLC-MS/MS. J. Guangdong Pharm. Univ. 35, 378–382. doi:10.16809/j.cnki.2096-3653.2019040101
Yang, J. Q., Li, Y. M., Yang, Y. F., Wu, T., Li, X. F., Kang, X. D., et al. (2020). Chemical Constituents of Callicarpa Nudiflora Leaves. J. Chin. Med. Mater. 1619–1623. doi:10.13863/j.issn1001-4454.2020.07.015
Yang, L., Meng, X., Yu, X., and Kuang, H. (2017). Simultaneous Determination of Anemoside B4, Phellodendrine, Berberine, Palmatine, Obakunone, Esculin, Esculetin in Rat Plasma by UPLC-ESI-MS/MS and its Application to a Comparative Pharmacokinetic Study in normal and Ulcerative Colitis Rats. J. Pharm. Biomed. Anal. 134, 43–52. doi:10.1016/j.jpba.2016.11.021
Yang, L. L., Xiao, N., Li, X. W., Fan, Y., Alolga, R. N., Sun, X. Y., et al. (2016). Pharmacokinetic Comparison between Quercetin and Quercetin 3-O-β-Glucuronide in Rats by UHPLC-MS/MS. Sci. Rep. 6, 35460. doi:10.1038/srep35460
Yi, J. P., Wu, Y. Z., Yu, N., Yu, Z. W., Xie, F. Y., and Yuan, Q. (2016). VEGF Gene Polymorphisms Affect Serum Protein Levels and Alter Disease Activity and Synovial Lesions in Rheumatoid Arthritis. Med. Sci. Monit. 22, 316–324. doi:10.12659/MSM.894912
Yin, Y. T., Guo, Q. X., Chen, F., and Hong, F. (2020). Overview on the Pharmacological Action of Tricin. Guangming J. Chin. Med. 35, 2105–2108. doi:10.3969/j.issn.1003-8914.2020.13.060
Zhan, J. S., Zhan, K., Chen, X. L., Huo, J. H., and Zhao, G. Q. (2018). Lipopolysaccharide-induced Effects of Tricin on Inflammatory and Lacto-Protein Gene Expression in Bovine Mammary Epithelial Cells. Prata. Sci. 35, 441–448. doi:10.11829/j.issn.1001-0629.2017-0184
Zhang, B., Wang, X., and Li, S. (2013a). An Integrative Platform of TCM Network Pharmacology and its Application on a Herbal Formula, Qing-Luo-Yin. Evid. Based Complement. Alternat Med. 2013, 456747. doi:10.1155/2013/456747
Zhang, L. N., Deng, R. X., Wang, Y., Liu, M. M., and Liu, P. (2019a). Chemical Constituents from Rhizome of Phlomis Umbrosa. Chin. Pharm. J. 54, 450–456.
Zhang, M. Y., Zhu, Z. M., Yao, D., Chen, Q. H., Bao, X. W., Chen, J. W., et al. (2019b). Simultaneous Detection of Cordycepin, D-Mannitol, Adenosine and Inosine in Cordyceps Militaris by HILIC-MS/MS. Chin. J. Bioproc. Eng. 17, 430–436. doi:10.3969/j.issn.1672-3678.2019.04.015
Zhang, X., Wang, L., Zheng, Z., Pi, Z., Liu, Z., and Song, F. (2017). Online Microdialysis-Ultra Performance Liquid Chromatography-Mass Spectrometry Method for Comparative Pharmacokinetic Investigation on Iridoids from Gardenia Jasminoides Ellis in Rats with Different Progressions of Type 2 Diabetic Complications. J. Pharm. Biomed. Anal. 140, 146–154. doi:10.1016/j.jpba.2017.03.040
Zhang, Y., Wang, K., Chen, H., He, R., Cai, R., Li, J., et al. (2018). Anti-inflammatory Lignans and Phenylethanoid Glycosides from the Root of Isodon Ternifolius (D.Don) Kudô. Phytochemistry 153, 36–47. doi:10.1016/j.phytochem.2018.05.017
Zhang, Z. J., Sun, D. X., Wang, J., Dong, L. H., Guo, P. P., and Wang, C. Y. (2013b). Simultaneous Determination of Eight Components in Kouyanqing Granules by LC-MS/MS. Chin. Tradit. Pat. Med. 35, 952–956. doi:10.3969/j.issn.1001-1528.2013.05.020
Zhao, Y. S., Bi, Y. Q., Lei, L. J., Zhu, X. H., Lv, Y., Zhang, C. H., et al. (2017). [Variety Systematization and Research Progress of Mongolian Medicine "Bashaga"]. Zhongguo Zhong Yao Za Zhi 42, 998–1004. doi:10.19540/j.cnki.cjcmm.20170121.027
Zheng, C., Wang, J., Liu, J., Pei, M., Huang, C., and Wang, Y. (2014). System-level Multi-Target Drug Discovery from Natural Products with Applications to Cardiovascular Diseases. Mol. Divers. 18, 621–635. doi:10.1007/s11030-014-9521-y
Zhou, H. Z., Luo, W., Chen, S., Chen, H. L., Li, G. Y., and Li, L. M. (2021). Coumarins from Flowers of Stellera Chamaejasme and Their Biological Activities. Chin. Tradit. Herb. Drugs 52, 943–950. doi:10.7501/j.issn.0253-2670.2021.04.006
Zhou, J., Wang, Q., Xiang, Z., Tong, Q., Pan, J., Wan, L., et al. (2019). Network Pharmacology Analysis of Traditional Chinese Medicine Formula Xiao Ke Yin Shui Treating Type 2 Diabetes Mellitus. Evid. Based Complement. Alternat Med. 2019, 1–15. doi:10.1155/2019/4202563
Keywords: Odontites vulgaris, rheumatoid arthritis, UPLC-MS/MS, network pharmacology, PI3k-Akt signal pathway, RAW 264.7
Citation: Ji M, Wang C, Yang T, Meng X, Wang X and Li M (2021) Integrated Phytochemical Analysis Based on UPLC–MS/MS and Network Pharmacology Approaches to Explore the Effect of Odontites vulgaris Moench on Rheumatoid Arthritis. Front. Pharmacol. 12:707687. doi: 10.3389/fphar.2021.707687
Received: 10 May 2021; Accepted: 16 August 2021;
Published: 30 August 2021.
Edited by:
Zhong-qiu Liu, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Imma Pagano, University of Salerno, ItalyJianxin Chen, Beijing University of Chinese Medicine, China
Copyright © 2021 Ji, Wang, Yang, Meng, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhui Li, prof_liminhui@yeah.net; Xiaoqin Wang, nywangxiaoqin@163.com
†These authors have contributed equally to this work
 Mingyue Ji1†
Mingyue Ji1† Congcong Wang
Congcong Wang Minhui Li
Minhui Li