Metabolite profiling of Bruguiera cylindrica reveals presence of potential bioactive compounds
- Published
- Accepted
- Received
- Academic Editor
- Julia Martín
- Subject Areas
- Analytical Chemistry (other), Mass Spectrometry
- Keywords
- Mangrove, Bruguiera cylindrica, GC-MS, UHPLC-Q-Exactive Orbitrap HRMS
- Copyright
- © 2022 Dahibhate and Kumar
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Analytical Chemistry) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Metabolite profiling of Bruguiera cylindrica reveals presence of potential bioactive compounds. PeerJ Analytical Chemistry 4:e16 https://doi.org/10.7717/peerj-achem.16
Abstract
Bruguiera cylindrica parts are commonly used in Chinese and Indian traditional medicine to treat diarrhea, fever, and many ailments. The present study aims non targeted analysis of key secondary metabolites of B. cylindrica by gas chromatography mass spectrometry (GC-MS) and ultra-high performance liquid chromatography hybrid quadrupole-Exactive-Orbitrap high resolution mass spectrometry (UHPLC-Q-Exactive Orbitrap HRMS). GC-MS and UHPLC-Q-Exactive Orbitrap HRMS were utilized for metabolic profiling of ethyl acetate extract of B. cylindrica leaves. Key metabolites in the extract were identified and predicted based on chemical similarity using online databases such as ChemSpider and mzCloud. Thirty-six compounds belonging to different classes of secondary metabolites viz. flavonoids, fatty acids, fatty acid amides, carboxylic acids, and alkaloids were identified in the extract. Pentacyclic triterpenes like betulin, ursolic acid and a tropine, an alkaloid with potential pharmacological and therapeutic activities such as anticancer properties, neuromuscular blockers and antioxidants, were also identified. This study combined GC-MS and UHPLC-Q-Exactive Orbitrap HRMS with available online database for effective and rapid identification of bioactive metabolites in the ethyl acetate extract of mangrove without individual standard application. This is the first report on the HRMS based secondary metabolic profiling of B. cylindrica, with comprehensive map of its biologically important metabolites.
Introduction
Mangroves are a unique ecosystem that found along the world’s sheltered intertidal coastlines as well as in tropical and subtropical regions (Kathiresan & Rajendran, 2005; Saddhe, Jamdade & Kumar, 2017). The mangrove forest ecosystem is extremely productive and evolutionary adapted to extreme environmental stress (abiotic and biotic stress) and produces structurally diverse metabolites in response to it. Mangroves are rich source of metabolites belonging to class of fatty acids, limonoids, phenolics, coumarins, lignans, terpenoids, and alkaloids (Du et al., 2007; Cui et al., 2005; Sadhu et al., 2006; Bao et al., 2007; Taniguchi et al., 2018; Wu et al., 2008) which shows that they can be taken in an account for the search of novel bioactive metabolites. Furthermore, metabolites produced by mangrove species such as, Avicennia marina, Acanthus ilicifolius, Xylocarpus granatum, Excoecaria agallocha, Kandelia candel and Rhizophora mucronata that may offer disease preventing or medicinal properties as antitumor, antifungal, antimicrobial, antiviral, anti-inflammatory, and insecticidal effect (Wu et al., 2008; Dahibhate, Saddhe & Kumar, 2019; Nabeelah Bibi et al., 2019; Mitra, Naskar & Chaudhuri, 2021).
Bruguiera cylindrica L. is an evergreen mangrove from Rhizophoraceae family widely distributed in Asia pacific region, Southeast Asia and Western part of India (Saddhe, Jamdade & Kumar, 2017). B. cylindrica has been used by the Thai people in folk medicine to treat diarrhea and wound healing. The different parts of B. cylindrica extract are used for the treatment of hemorrhage, ulcers (Nithyamol et al., 2018), antimicrobial (Vadlapudi & Naidu, 2009; Dahibhate, Saddhe & Kumar, 2019; Dahibhate, Roy & Kumar, 2020), antioxidant (Krishnamoorthy et al., 2011), antiviral (Premanathan, Kathiresan & Nakashima, 1999), thrombolytic (Vadlapudi & Naidu, 2009) and anti-inflammatory (Eldeen, Ringe & Ismail, 2019). Research on B. cylindrica and other Bruguiera species are mainly concentrated on qualitative phytochemical screening whereas, gas chromatographic studies of different solvent extracts have shown that B. cylindrica is the valuable source of fatty acids, flavonoids, tannins, terpenes, alkaloids, glycosides and so on (Revathi, Jeyaseelansenthinath & Thirumalaikolundhusubramaian, 2015; Dahibhate, Roy & Kumar, 2020). Additionally, sulfur containing compounds and pharmacologically active triterpenoids are identified from fruits and, leaves of various other Bruguiera genus. These terpenoids mainly comprise beyerane, pimarane, lupane, ursane, oleanane, and dammarane (Wang et al., 2018; Jun et al., 2008; Nebula, Harisankar & Chandramohanakumar, 2013). These terpenes and other metabolites are focused due to structural diversity, and a wide range of bioactivities with cytotoxic effect against lung cancer and sarcoma cells, in vitro (Sithranga & Kathiresan, 2010). Therefore, in order to enhance the quality check of medicinal properties of plant and its effective utilization, an accurate and reliable analysis approach for monitoring the plant’s metabolite profile, which determines medicinal value, is required.
Metabolite profiling is one of the most recent approaches to the ‘omics’ revolution, which can provide a system-wide view of metabolite involved. It can elucidate complex processes by analyzing a large number of metabolites involved in numerous biochemical processes and across a wide range of biological systems. In plants, metabolite profiling is mainly performed by analytical platforms such as gas chromatography (GC), liquid chromatography (LC), capillary electrophoresis (CE) coupled to mass spectrometry (MS) and nuclear magnetic resonance (NMR) (Wang et al., 2015). It is challenging and time consuming to separate and identify every single metabolite in natural medicinal plants due to their complicated compositions. GC-MS is the most frequently used technology for plant metabolite, especially for identifying and quantifying metabolites involved in primary metabolism’s core pathways, such as sugars, amino acids, and organic acids. Several studies have reported the use of GC-MS technique for fatty acids determination in animals, marine organisms, plants, flax seeds, dietary fatty acid, and quality analysis of edible oils (Seppänen-Laakso, Laakso & Hiltunen, 2002; Tang & Row, 2013; Stenvers, Chi & Javidpour, 2020; Schött et al., 2021). Due to the lower boiling point than other metabolites fatty acids are easier to identify with GC-MS (Seppänen-Laakso, Laakso & Hiltunen, 2002). On the other hand, Ultra high performance liquid chromatography-Q-Exactive Orbitrap tandem mass spectrometry (UHPLC-Q-Exactive Orbitrap HRMS) technology combines the separation capabilities of chromatography with the qualitative functions of mass spectrometry. It has a high separation, scanning speed, resolution sensitivity, and compatibile with available natural product databases. UHPLC-Q-Exactive Orbitrap HRMS is the most widely used technique for analyzing fatty acids, polyphenolics, alkaloids, and terpenes category secondary metabolite from complex system like plants (Li et al., 2021; Ossipov et al., 2020; Liu et al., 2020). Additionally, this technique is especially suitable for the qualitative identification of the natural products and structural analysis of the novel compounds lacking the standard reference metabolite.
On the basis of our literature survey, no metabolic profiling studies have been reported from B. cylindrica of West coast India. However, metabolite profiling has not been done so far on B. cylindrica leaves by ultra-high performance liquid chromatography hybrid quadrupole-Orbitrap high resolution mass spectrometry (UHPLC-Q-Exactive Orbitrap HRMS). In fact, the leaves are a renewable and long-term source of secondary metabolites which are important for long term survival and defense against biotic stress. In the present study, to explore the major secondary metabolites of B. cylindrica leaves having various pharmacological activities, we used the GC-MS and UHPLC-Q-Exactive Orbitrap HRMS (Thermo Scientific, Waltham, MA, USA) coupled to online database (NIST, ChemSpider and mzCloud) to assess the metabolite composition of ethyl acetate extract from B. cylindrica leaves.
Materials and Methods
Chemicals and reagents
Methanol, acetonitrile, formic acid, of LCMS grade (Thermo Fisher Scientific, Waltham, MA, USA), ethyl acetate, and water from Milli-Q purification unit (Merck, Merck, Germany) were obtained.
Plant collection and treatment
In present study, leaf samples of B. cylindrica were collected from Goa, located on India’s west coast at geographical latitude of 15.5256°N and a longitude of 73.8753°E. B. cylindrica was identified based on morphological keys (Saddhe, Jamdade & Kumar, 2016, 2017) and voucher specimens were maintained. The leaves were collected from the single sampling location in the month of August 2021. Leaves were washed, dried under shed at 30 °C and crushed using electric grinder. A grounded sample weighed (100 gm), packed in cellulose thimble and extracted with ethyl acetate (3 × 150 mL) using Soxhlet apparatus operated at 50 °C for 24 h. Extract was filtered using cellulose filter paper (125 mm; GE Healthcare, Chicago, IL, USA), concentrated using rotary evaporation (Medica Instruments, Mumbai, India) and kept at −20 °C until further analysis.
Sample preparation
Prior to analysis, parent stock was prepared by dissolving 10 mg of sample in one mL of ethyl acetate and heated in water bath (50 °C) to ensure its complete dissolution and then centrifuged (10,000 rpm, 8 min). For GC-MS analysis, sample was prepared by adding a volume of 10 µL from parent stock to 990 µL of ethyl acetate (v/v). For UHPLC-Q-Exactive Orbitrap HRMS analysis, final working stock of 1 mg/mL was prepared in an equal volume of ethyl acetate: methanol (v/v) and filtered through 0.22 µm nylon membrane filter (GE Healthcare, Chicago, IL, USA) before injection. The analysis was performed in three independent technical replicates.
Gas chromatography mass spectrometry (GC-MS) analysis
The ethyl acetate extract of B. cylindrica was analyzed using GC-MS system of Shimadzu (GC-2030) series equipped with headspace (HS-20) & triple quadrupole mass spectrometer GC-TQ8040NX. The fused silica column, SH-Rxi-5 (0.25 mm × 30 m, 0.25 µm) was used. Carrier gas (Helium) was used with a flow rate of one mL/min. The column temperature was maintained at 50 °C for 3 min and increased to 130 °C at the rate of 7 °C for 4 min, followed by increased to 250 °C at the rate of 7 °C for 3 min, through split ratio (50:50) mode. Injector, ion source, and interface temperature were set to 260, 220 and 260 °C respectively. For analysis 1 µL of the sample was injected with a constant temperature of 260 °C through an autosampler injector. The ionization energy was 70 eV and mass range of 40–500 amu. The system management, mass spectrometry, parameter settings, data receipt and processing were performed using Shimadzu real time analysis. Identification of metabolite constituents was made on the basis of retention, MS library search in National Institute of Standards and Technology (NIST 17) and comparing with literature data.
UHPLC-Q-Exactive Orbitrap HRMS analysis
Qualitative analysis of ethyl acetate extract of B. cylindrica was acquired using UHPLC system (Thermo Scientific, Chicago, IL, USA) coupled to Q-Exactive Orbitrap MS system equipped with electrospray ionization. Hypersil gold (100 × 2.1 mm, 3.0 µm) column was used for the analysis. The column temperature was maintained at 28 °C and the injection volume was 5 µL. Mobile phase consists of water (0.1% formic acid) as phase A and acetonitrile as phase B with 0.3 mL/min of flow rate. Linear gradient elution parameters were set as follows: 0–2 min 5% B and increased from 5% to 10% B in 1 min. Further in 20 min, the gradient was linearly increased to 95% B and returned to 5% B in 6 min and re-equilibrated for 4 min at 5% B. The blank injection was employed after each sample to avoid the carryover.
Mass spectrometry parameters were as follows; full scan data was acquired in both positive and negative mode at resolving power of 70,000 with 100–1,500 scanning range. Ion source parameter were: auxiliary gas 9 (N2, 95%), sheath gas 37, spray voltage set to 3 kV and capillary temperature and auxiliary gas heater were at 300 °C and 350 °C respectively. The S-lens RF level was set at 55. The automatic gain control was set at 1 × 106 and injection time was set at 60 ms. Scan rate was 1 scans/s. The product ion spectra were obtained in the range of 25–40 eV of collision energy. Mass tolerance was set to 5 ppm. Data acquisition was performed using the Thermo Scientific Xcalibur software (version 4.2.48.14). Compound Discoverer software 2.1 SP1 (Thermo Fisher Scientific, Chicago, IL, USA), a qualitative data-processing application that uses accurate mass data and mass spectral library searches for the small molecule identification, was used for data processing. Both spectral libraries and compound databases like mzCloud and ChemSpider were used for metabolite identification. The only those identified metabolites which are consistently appeared over three injections are reported.
Results and Discussions
Extraction of bioactive compound
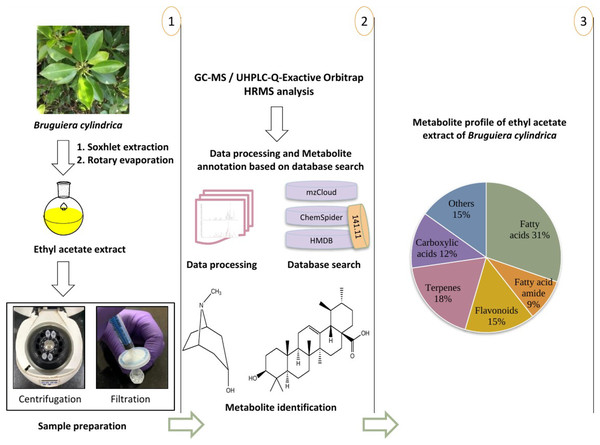
In fact, the leaves are a renewable and crucial source of plant secondary metabolites which are important for long term survival and defense against biotic stress, therefore can be selected for screening of active metabolites. The Soxhlet extraction, also known as the hot continuous extraction process and its fundamental advantages are simplicity, allows thorough extraction with the least amount of solvent at high temperature, which ultimately improves the process kinetics. The disadvantage of this approach is that it is not ideal for the extraction of thermo-labile metabolites since prolonged heating may cause the compounds to degrade. In our previous research, we have employed Soxhlet apparatus using ethyl acetate for the extraction of biologically important metabolites and metabolic profiling studies (Dahibhate, Roy & Kumar, 2020; Dahibhate, Kumar & Kumar, 2021). Additionally, as a suitable solvent, ethyl acetate was selected because of its chemical and biological characteristics including medium polarity and minimum toxicity. Ethyl acetate biphasic nature enables to extract both polar and non polar biological compounds. Therefore, we selected ethyl acetate as extraction solvent and the extract was processed for the metabolic profiling using GC-MS and UHPLC-Q-Exactive Orbitrap HRMS technique. Figure 1 shows typical metabolite profiling workflow including experimental design and preparation of samples to metabolite annotation.
Figure 1: Metabolite profiling workflow including experimental design, preparation of samples and metabolite annotation.
GC-MS profiling revealed presence of 11 metabolites
GC-MS profiling of the extracts showed presence of compounds from different classes with key biological properties. The compounds were identified by relating their retention time, retention index, peak area (%) and mass spectral fragmentation pattern with known metabolites library of NIST. From all the peaks observed in the ethyl acetate extract, only 11 compounds were identified with more than 89% similarity index, which is listed in Table 1. The GC-MS analysis of ethyl acetate of B. cylindrica has revealed the presence of lauric acid, myristic acid, neophytadiene, phytol, palmitic acid, 10(E),12(Z)-octadecadienoic acid, oleic acid, stearic acid, nonacosane, tetracontane and squalene. The squalene, a triterpene (22.69%) was determined as a major component of extract followed by palmitic acid (15.54%), oleic acid (12.71%), and neophytadiene, a diterpene (5.17%). Besides, straight chain hydrocarbon such as, tetracontane, nonacosane content was higher than those of saturated fatty acids such as lauric acid, stearic acid and myristic acid.
| Rt (min) | Compound name | Molecular formula | Molecular weight | Base m/z | Similarity (%) | Retention index | Area (%) | Class |
|---|---|---|---|---|---|---|---|---|
| 24.947 | Lauric acid | C12H24O2 | 200 | 73 | 94 | 1,570 | 1.28 | Fatty acid |
| 28.825 | Myristic acid | C14H28O2 | 228 | 73 | 96 | 1,769 | 0.82 | Fatty acid |
| 29.996 | Neophytadiene | C20H38 | 278 | 68 | 96 | 1,774 | 5.17 | Diterpene |
| 30.683 | Phytol | C20H40O | 296 | 81.05 | 93 | 2,045 | 1.95 | Diterpene |
| 32.048 | Palmitic acid | C16H32O2 | 256 | 73 | 96 | 1,968 | 15.54 | Fatty acid |
| 34.426 | 10(E),12(Z)-octadecadienoic acid | C18H32O2 | 280 | 67 | 94 | 2,183 | 3.94 | Fatty acid |
| 34.514 | Oleic acid | C18H34O2 | 282 | 55 | 92 | 2,175 | 12.71 | Fatty acid |
| 34.798 | Stearic acid | C18H36O2 | 284 | 73 | 89 | 2,167 | 4.56 | Fatty acid |
| 36.023 | Nonacosane | C29H60 | 408 | 57.05 | 95 | 2,904 | 4.4 | Hydrocarbon |
| 39.384 | Tetracontane | C40H82 | 562 | 57 | 96 | 3,997 | 5.41 | Hydrocarbon |
| 39.727 | Squalene | C30H50 | 410 | 69.05 | 97 | 2,914 | 22.69 | Triterpene |
Note:
Retention index derived from the NIST 17 database match.
Our results are in correlation with previous studies on B. cylindrica, in which palmitic and stearic acid was reported as dominant metabolites of B. cylindrica (Revathi, Jeyaseelansenthinath & Thirumalaikolundhusubramaian, 2015). Some of the compounds included in Table 1 have previously been reported for their role in various pharmaceutical and therapeutic functions (Casillas-Vargas et al., 2021). Essential oils rich in terpenes are like squalene, neophytadiene has been shown to posses ability to reduce free radical damage of skin (Huang, Lin & Fang, 2009), antimicrobial activity, analgesic and anti-inflammatory (Swamy et al., 2017). The dissimilarity with previous report (Revathi, Jeyaseelansenthinath & Thirumalaikolundhusubramaian, 2015) were observed in current study which may be due to seasonal variation, difference in sample collection, or experimental method employed for analysis.
UHPLC-Q-Exactive Orbitrap HRMS profiling of B. cylindrica revealed presence of 25 metabolites
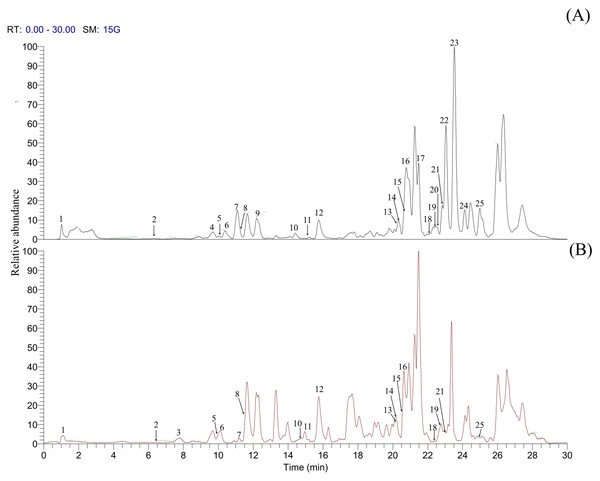
In the present study, a high-throughput and sensitive method of UHPLC coupled with Q-Exactive Orbitrap HRMS was used to identify the chemical constituents in B. cylindrica. By comparing with available secondary metabolite database such as chemSpider, mzCloud and available literature, 25 metabolites were confirmed from B. cylindrica, which have previously been shown to have various bioactive properties. Also, the identified metabolites were confirmed based on the accurate mass and theoretical fragmentation pattern matching criteria using the HMDB structural database (https://hmdb.ca/) and mass bank database (https://massbank.eu/MassBank/). All the identified metabolite information is summarized in Table 2. The base peak chromatogram of ethyl acetate extract obtained in the positive and negative mode under the optimized UHPLC-Q-Exactive Orbitrap HRMS conditions is shown in Fig. 2. Total 25 secondary metabolites were identified such as five flavonoids, three fatty acid amide, three terpenes, one alkaloid, four carboxylic acids, five fatty acids and four other components. Among these components flavonoids and fatty acids was found to be the major component of B. cylindrica.
| Peak | Rt (min.) | Compound name | Molecular formula | Calculated mass | Error (ppm) | mzCloud match score | ChemSpider match | Class | Biological activities | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.015 | Tropine | C8H15NO | 141.11516 | −1.45 | 98.2 | Full match | Alkaloid | Bradycardic, and respiratory-stimulant action | Mitra, Naskar & Chaudhuri (2021) |
| 2 | 6.499 | 4-Hydroxybenzaldehyde | C7H6O2 | 122.0366 | −1.47 | 96 | Full match | Aldehyde | Anti-angiogenic and anti-nociceptive activities | Lim et al. (2008) |
| 3 | 7.546 | Esculetin | C9H6O4 | 178.02637 | −1.35 | 88.9 | Full match | Coumarin | Anti-proliferative and pro-apoptotic activity | Jeon et al. (2015) |
| 4 | 9.654 | Syringic acid | C9H10O5 | 198.05251 | −1.6 | 75.8 | Full match | Carboxylic acid | Prevention of diabetes, neuro and hepatoprotective, | Tanase, Coșarca & Muntean (2019) |
| 5 | 10.015 | 4-Coumaric acid | C9H8O3 | 164.04717 | −1.08 | 84.7 | Full match | Carboxylic acid | Antivirus, anti-inflammatory | Tanase, Coșarca & Muntean (2019) |
| 6 | 10.17 | Dioctyl phthalate | C24 H38O4 | 390.27626 | −1.91 | 90.7 | Full match | Phthalates | – | – |
| 7 | 11.159 | Isoorientin | C21H20O11 | 448.09972 | −1.88 | 98.2 | Full match | Flavones | Anti-nociceptive, and gastroprotective activities | Panche, Diwan & Chandra (2016) and Kumar & Pandey (2013) |
| 8 | 11.437 | Orientin | C21H20O11 | 448.09972 | −1.88 | 93.5 | Full match | Flavones | Anti-nociceptive, and gastroprotective activities | Panche, Diwan & Chandra (2016) and Kumar & Pandey (2013) |
| 9 | 12.921 | Azelaic acid | C9H16O4 | 188.10501 | 0.8 | 95.1 | Full match | Carboxylic acid | Anti-infective and anti-inflammatory action | Schulte, Wu & Rosen (2015) |
| 10 | 14.774 | Luteolin | C15H10O6 | 286.0472 | −1.89 | 98.5 | Full match | Flavones | Inhibit angiogenesis, induce apoptosis. | Lopez-Lazaro (2009) |
| 11 | 15.194 | Kaempferol | C15H10O6 | 286.0472 | −1.69 | 90.6 | Full match | Flavonols | Antimicrobial, cardioprotective, neuroprotective, | Panche, Diwan & Chandra (2016) |
| 12 | 15.628 | Apigenin | C15H10O5 | 270.05226 | −2.07 | 96.5 | Full match | Flavones | Block cell cycle and decrease prostate cancer cells | Panche, Diwan & Chandra (2016) |
| 13 | 20.336 | 12-oxo Phytodienoic acid | C18H28O3 | 292.20324 | −1.17 | 77.5 | Full match | Carboxylic acid | – | – |
| 14 | 20.38 | Caryophyllene oxide | C15H24O | 220.18247 | −1.13 | 80.6 | Full match | Sesquiterpenoid | Antifungal, Analgesic and anti-inflammatory activity | Dahham et al. (2015) |
| 15 | 20.51 | Monobutyl phthalate | C12 H14O4 | 222.8899 | −0.98 | 86.2 | Full match | Phthalates | – | – |
| 16 | 20.772 | 9-Oxo-10(E),12(E)-octadecadienoic acid | C18H30O3 | 294.21898 | −1.75 | 96.5 | Full match | Fatty acid | Inhibits triglyceride accumulation | Kim et al. (2011) |
| 17 | 21.709 | Ursolic acid | C30H48O3 | 456.36079 | 0.98 | 98.1 | Full match | Triterpene | Anticancer, antidiabetic, antioxidant | Mlala et al. (2019) |
| 18 | 22.316 | Betulin | C30H50O2 | 442.38072 | −0.81 | 85.6 | Full match | Triterpene | Antitumor | Hordyjewska et al. (2019) |
| 19 | 22.747 | Hexadecanamide | C16H33NO | 255.25582 | −1.56 | 99 | Full match | Fatty acid amide | – | – |
| 20 | 22.732 | Linoleic acid | C18H32O2 | 280.24052 | 1.04 | 94.6 | Full match | Fatty acid | Affects peptidoglycan synthesis | Casillas-Vargas et al. (2021) |
| 21 | 22.956 | 9-Octadecenamide | C18H35NO | 281.27147 | −1.4 | 75.6 | Full match | Fatty acid amide | Increase cholinesterase and cholinesterase trasferase activity | Boger et al. (1998) |
| 22 | 23.048 | Palmitic acid | C16H32O2 | 256.24049 | 1.02 | 96.5 | Full match | Fatty acid | Anti-quorum sensing against Acinetobacter baumannii | Casillas-Vargas et al. (2021) |
| 23 | 23.229 | Oleic acid | C18H34O2 | 282.25612 | 0.84 | 94.9 | Full match | Fatty acid | Antioxidative effect | Wei et al. (2016) |
| 24 | 23.743 | Stearic acid | C18H36O2 | 284.27174 | 0.74 | 95.9 | Full match | Fatty acid | Antibacterial activity | Casillas-Vargas et al. (2021) |
| 25 | 24.969 | Docosanamide | C22H45NO | 339.34947 | −1.89 | 81.8 | Full match | Fatty acid amide | – | – |
Figure 2: Base peak chromatogram of the metabolites detected using UHPLC-Q-Exactive Orbitrap HRMS.
(A) positive mode and (B) negative mode.Flavonoids are ubiquitously distributed among plants in flavone or flavonol form and considered as an essential component in nutraceutical, medicinal, cosmetic application (Panche, Diwan & Chandra, 2016), and have anti-oxidant, anti-inflammatory, and anti-tumor properties (Lopez-Lazaro, 2009). In UHPLC-Q-Exactive Orbitrap HRMS analysis of B. cylindrica four flavone viz. two C-glycosidic flavone of luteolin (isoorientin, orientin), and two aglycone form (luteolin, and apigenin) were detected. Isoorientin and orientin were identified with precursor ion at m/z 449.1078. Differences in relative abundances of products ions m/z [M+H-60]+ and [M+H-120]+ were demonstrated as characteristic ions of C-glycosides, which could be used for identification (Pereira, Yariwake & McCullagh, 2005). Distinction of isomer pair of isoorientin and orientin was performed according to the relative abundance of product ion fragments such as, m/z 329.0653 [M+H-60]+ and m/z 299.0548 [M+H-120]+. The bioactive flavonols such as kaempferol showed precursor ion at m/z 286.04720.
Carboxylic acids are compounds occurring naturally in different stages of plants. They have a beneficial effect on microbial growth by acting as vitamin for microbial nutrition (e.g. nicotinic acid, or p-aminobenzoic acid). There are several studies reported about the significance of medicinal effects of carboxylic acid containing metabolites in treatment of pain and disease (Matsumoto, Yanagi & Oe, 2018). In this study, carboxylic acids like azelaic acid, 12-oxo-phytodienoic acid, syringic acid, 4-coumaric acid were detected. The 12-oxo-phytodienoic acid, a precursor of jasmonic acid in plants showed protonated parent ion [M+H]+ at m/z 293.2136. Further fragmentation resulted into product ions at m/z 275.200, 247.2051, 229.1947, 163.1115, and 149.0231. There is currently no literature available on the mass fragmentation of 12-oxo-phytodienoic acid available, hence we experimentally confirmed the fragments formed with perfect match using predicted MS/MS spectra from The Human Metabolome Database. Additionally, syringic acid, azelaic acid and 4-coumaric acids showed precursor ion at m/z 199.5301, 189.11021 and 165.04877 respectively. Moreover, it has been reported that syringic acid and 4-coumaric acids are found to be most effective as providing anti-oxidant, anti-virus, gastroprotective activities (Tanase, Coșarca & Muntean, 2019; Gomez et al., 2019) but current knowledge regarding the biological activities of 12-oxo-phytodienoic acid is still far from complete. Our findings showed presence of fatty acid such as, linoleic acid, plamitic acid, oleic acid, stearic acid and 9-oxo-10(E),12(E)-octadecadienoic acid in B. cylindrica. The recent study from tomato plant reported the effect 9-oxo-10(E), 12(E)-octadecadienoic acid on triglyceride accumulation by acting agonist of peroxisome proliferator-activated receptor α on mouse primary hepatocytes (Kim et al., 2011).
The published data shows that Bruguiera genus has been well known for the presence of different types of di- and triterpenoids like beyerane, pimarane, lupane, ursane, oleanane, and dammarane (Wang et al., 2018; Jun et al., 2008). Nithyamol et al. (2018) reported a pentacyclic triterpene, a taraxerol is the main metabolite from various solvent fractions of Bruguiera cylindrica. In our work we searched for the taraxerol in analyzed samples but unfortunately we do not found it in single ion monitoring mode and through selective extraction of ion chromatogram as well. This variation in the metabolite produced which may be due to change in the geographical locations, as both the sampling studies are conducted from different part of India. Additionally, our study confirms the presence of one sesquiterpene, caryophyllene oxide and two pentacyclic triterpene, a ursane type ursolic acid (m/z 457.3702) and lupane type betulin (m/z 443.3883) using accurate mass measurement and fragmentations. The presence of an intense signal of product ions with m/z 191, 217, 335 were in correlation with reported literature for betulin (Kosyakov, Yanovskii & Falev, 2014). It has been studied extensively because of its remarkable biological properties like anti-melanoma and anti-HIV activities (Mitra, Naskar & Chaudhuri, 2021; Hordyjewska et al., 2019). Other compound belongs to nitrogenous bicyclic organic compound category such as tropine, which shown peak at m/z 142.1225. The tropine was characterized in B. exaristata and reported to have respiratory-stimulant action (Nebula, Harisankar & Chandramohanakumar, 2013; Mitra, Naskar & Chaudhuri, 2021). Furthermore, esculatin (m/z 179.0376) and 4-hydroxybenzaldehyde (m/z 123.0421) were detected as well.
Fatty acid amides (FAAs) are family of structurally diverse molecules formed from a fatty acid, and an amine is well studied in relevance to human than to other organisms. In this study, we have detected three different FAAs, namely 9-octadecenamide (m/z 282.3279), hexadecanamide (m/z 256.3282), and docosanamide (m/z 340.3988). 9-octadecenamide is well studied molecule among FAAs, which is derived from fatty acid oleic acid. It has a role as plant metabolite as well as human metabolite known for inhibiting gap junction communication in the glial cells (Boger et al., 1998). Previously, Cryptotaenia japonica was reported to have higher content of 9-octadecenamide, which function is attributed to superoxide scavenging and hypolipidemic bioactive property (Cheng et al., 2010). Whereas oleamide, hexadecamide along with other FAAs like stearamide, erukamide has been recently reported in Artemisia argyi (Cui et al., 2021). These compounds have been reported first time in B. cylindrica by UHPLC-Q-Exactive Orbitrap HRMS.
Phthalates or esters of phthalic acid are widely used to make the plastic softer and emitted into the environment during manufacturing, use, and disposal (Thiemann, 2021). With an exception to our main objective of the study, UHPLC-Q-Exactive Orbitrap HRMS analysis of B. cylindrica also reveals the presence of two monobutyl (m/z 223.0984) and dioctyl phthalates (m/z 391.3102), which might be associated with contamination of mangrove water bodies. In addition to this, da Silva Pontes et al. (2020) has recently carried systematic GC-MS based study to examine the accumulation of phthalate in mangrove Avicennia schaueriana from Sundarban. Surprisingly, bis-isobutyl phthalate, bis-tridecyl phthalate, bis-2-ethylhexyl phthalate were the major metabolites detected. On the other hand, phthalates have continued to be reported as endogenous product from plants, and microorganisms (Romeh, 2013; Zhang et al., 2018). Contrarily, it remains as puzzle, whether the phthalates should be considered as natural product or contaminants of environmental pollution (Thiemann, 2021).
In comparison to high-performance liquid chromatography (HPLC), it is advantageous to use UPLC by considering short time of analysis, improved sensitivity with triple quadrupole. UPLC is often hyphenated with different sources of ionization and mass detectors has made it a convenient technique for analysis of complex mixtures, and also proved its prime role in exploring the constituents of traditional medicinal plants (Alvarez-Rivera et al., 2019). UPLC-Q-Orbitrap-MS/MS and GC-MS are currently commonly used for the metabolite profiling of different plants. In contrast to the traditional LC-ESI-MS, UPLC-Q-Orbirap-HRMS has higher resolution, selectivity and eliminate sample matrix interference. Moreover, it was reported that the secondary metabolites identified by GC-MS and UHPLC-Q-Exactive Orbitrap HRMS in this study possess group of bioactivities of therapeutic interest (Kumar et al., 2009; Casuga, Castillo & Corpuz, 2016). The chemical constituents reported in this study were relatively inclusive, and first report on UHPLC-Q-Exactive Orbitrap HRMS based analysis of B. cylindrica. As there are not enough research articles about GC-MS and UHPLC-Q-Exactive Orbitrap HRMS based analysis of B. cylindrica to correlate the current finding of this report, hence this work can be considered as foundation for further studies on B. cylindrica.
Conclusions
Current study discussed about the identification and characterization of secondary metabolites in ethyl acetate extracts from B. cylindrica leaves. In total, 11 compounds were identified by GC-MS and 25 chemical constituents of B. cylindrica were identified first time by UHPLC-Q-Exactive Orbitrap HRMS technology. Moreover, these HRMS, GC-MS platform could be used for the evaluation of the similarities between medicinal plant extracts and their commercial products. Previously, qualitative and in vitro studies on B. cylindrica have shown primarily phenols, sterols, diterpenoid, triterpenoids, and fatty acids. Our data confirmed the presence of flavones, fatty acids, terpenes, and carboxylic acids. This study can be considered as foundational work about metabolite analysis of B. cylindrica, which may provide the substructure for metabolite profiling, characterization and bioactive properties of B. cylindrica and other species of Bruguiera genus.