Abstract
Seeds of bottlebrush, a novel plant material, were found to exhibit excellent adsorption capacity over a wide range of Cd(II) concentration. It was characterized by Fourier transform infrared spectroscopy and Scanning Electron Microscopy to support the adsorption of Cd(II) ions. Effect of various parameters like pH, contact time, initial concentration and different electrolytes was investigated using batch process to optimize conditions for maximum adsorption. The adsorbent data were analyzed using Langmuir, Freundlich, Temkin and Dubinin–Redushkeuich isotherm equations at 30°, 40° and 50 °C. Thermodynamic parameters such as standard enthalpy change (ΔH°), free energy change (ΔG°) and entropy change (ΔS°) were also evaluated and the results indicated that adsorption of Cd(II) are spontaneous and endothermic. Various kinetics models including the Pseudo-first-order kinetics, Pseudo-second-order kinetics and Intraparticle diffusion models have been applied to the experimental data to predict the adsorption kinetics. Kinetic study was carried out by varying initial concentration of Cd(II) at constant temperature and it was found that pseudo-second-order rate equation was better obeyed than pseudo-first-order equation supporting that chemisorption process was involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water pollution is one of the most serious environmental problems being faced by modern society (Marques et al. 2000; Ruiz-Manriquez et al. 1998). Heavy metals are known for their non-degradability and toxicity. It is, therefore, essential to control their discharge into water bodies and natural streams. Cadmium is highly toxic and non-essential metal which is released into the environment by various ways such as photographic development, ceramic, alkaline batteries, electroplating and metal plating works (Mohapatra and Anand 2007). Cadmium exposes human health to severe risks, due to its higher toxicity and can exert its toxic effects even at low concentrations. Occupational levels of cadmium exposure are a risk factor for chronic lung disease and testicular degradation (Benoff et al. 2000). Cadmium can also damage some specific structure of the functional units of the kidney (Satarug et al. 2000). Cadmium concentrations in unpolluted natural water are usually below 0.001 mg L−1 (Friberg et al. 1986) therefore it is necessary to remove cadmium from industry effluent. For this reason, it is significant to focus the attention on the development of improved friendly methods and low-cost adsorbents for toxic heavy metal removal. Various techniques are in use for the removal of heavy metals from the industrial discharge but adsorption technology has been found to show high efficiency in detoxifying effluents (Kenduzler and Turker 2005). Adsorption utilizes the ability to accumulate heavy metals from wastewater by either metabolically medium or physiochemical pathway of uptake (Fourest and Roux 1992). Natural materials have excellent potential as inexpensive adsorbents. Many adsorbents have been used in past years for cadmium ion removal, however, the exploration and development of new adsorbents is endless. Biomaterials such as Cashew nut shell (Kumar et al. 2012a, b), Borassus aethiopum seed shells (Adie et al. 2012), Modified corncob (Leyva-Ramos et al. 2012), Chitosan (Hydari et al. 2012), Agricultural wastes (Chao and Chang 2012), Seeds of Mast tree (Rao and Rehman 2012), Peganum harmala seeds (Zamani et al. 2013), Nylon-6 (Prakash et al. 2012), Calcite sludge (Merrikhpour and Jalali 2012), Rice straw (Ding et al. 2012), Meranti wood (Rafatullah et al. 2012), Nauclea diderrichii seed (Omorogie et al. 2012), Modified Lawny grass (Chen et al. 2011), Acacia nilotica (Waseem et al. 2012), Walnut shell (Almasi et al. 2012), Grapefruit biomass (Bayo et al. 2012), Macrocystis pyrifera (Cazon et al. 2012), Algae (Sulaymon et al. 2012), Modified Cashew nut shell (Kumar et al. 2012a, b), Rice bran (Chen et al. 2012), Sawdust (Bouziane et al. 2012), Orange peel (Masoudi et al. 2012) etc. have recently been utilized for the removal of Cd(II) from aqueous solution.
Bottlebrush plants (Callistemon chisholmii) are medium size trees or bush with flowers that resemble a bottlebrush because of their cylindrical brush like flower. This plant belongs to Callistemon species and Myrtaceae family and mostly found in the temperate region of Australia. It is generally available in many areas within its hardiness range (Gilman and Watson 1993). The leaves of this plant are a substitute of tea and have a delightfully refreshing flavour (Cribb and Cribb 1976). Its leaves when crushed emit a refreshing scent like lemon (Genders 1977). Flowering of bottlebrush is normally spring and early summer (Gilman and Watson 1993). Flower head are mostly red in colour but some are green, yellow or white. Each of them produces a triple-celled seed capsules around the stem.
This work has been undertaken to explore the adsorption behaviour of this promising novel material (bottlebrush seed capsules) towards Cd(II) ions. The effect of parameters such as initial solution pH, heavy metal concentration, and contact time was examined. Several characterization techniques (FTIR, SEM) were also used to identify the changes in bottlebrush seeds to determine its applicability in the adsorptive removal of Cd(II). This material has not yet been tested for the adsorption of heavy metals from aqueous solution and therefore its excellent adsorption capacity, ease of availability, non-toxic nature, cheapness etc. give the main innovation of the present study.
Materials and methods
Preparation of the adsorbent
Seed capsules of bottlebrush were crushed, sieved and washed with double distilled water (DDW) to remove dust and dirt etc. Capsules were dried and then sieved to 150–300 μm size. The procedure used to prepare the adsorbent referred to previous works (Rao et al. 2012; Rao and Kashifuddin 2012a, b). The material was placed in an airtight container for further use.
Adsorbate solution
Stock solution of cadmium (1,000 mg L−1) was prepared by dissolving desired quantity of their nitrate salt, Cd (NO3)2·H2O (A.R. Grade) in double distilled water (DDW).
Adsorption studies
Adsorption studies were carried out by batch process. The amount of 0.5 g adsorbent was placed in a conical flask in which 50 mL Cd(II) solution of desired concentration was added in a 250-mL conical flask and the mixture was shaken in temperature-controlled shaker incubator for 24 h. The mixture was then filtered using Whatman filter paper no. 41 and final concentration of metal ion was determined in the filtrate by Atomic Absorption Spectrophotometer (GBC-902, Australia). The amount of Cd(II) adsorbed was calculated by subtracting final concentration from initial concentration.
Characterization of adsorbent
Scanning Electron Microscope (Ametek, USA) was used to identify the surface quality and morphology of the adsorbent at an accelerating voltage of 20 kV. To identify the binding groups present before and after adsorption on the adsorbent surface and their involvement in adsorption process, Fourier Transform Infrared Spectroscopy (FTIR) were recorded on PerkinElmer FTIR Spectrum BX (PerkinElmer Life and Analytical Sciences, CT, USA) using KBr pellets in the ratio of 1:100 and spectra recorded in the range of 400–4,000 wave number (cm−1).
Effect of temperature
The effect of temperature on the adsorption of Cd(II) was studied by varying the adsorbent doses from 0.1 to 1.0 g at fixed volume (50 mL) of the Cd(II) solution with initial concentration 50 mg L−1 initial concentration. These flasks were kept into temperature-controlled water bath shaker at different temperatures (30–50 °C) for 4 h and then filtered. The final concentration of the desired compound in the filtrate from each flask was then determined as described earlier.
Effect of pH
The volume of 50 mL Cd(II) solution containing 50 mg L−1 Cd(II) was taken in a beaker and the pH of the solution was adjusted by adding 0.1 M HCl or 0.1 M NaOH solution using (ELICO—Li, India) pH meter. The solution was taken in conical flak, treated with 0.5 g of the adsorbent and after the attainment of equilibrium, the final concentration of Cd(II) was determined. The % adsorption was then calculated by subtracting final concentration from initial concentration. The final or equilibrium pH pHf) was also recorded. To investigate the effect of electrolyte, the same procedure was repeated with Cd(II) solution (50 mg L−1) prepared in 0.5 M KNO3.
Point of zero charge
The point of zero charge (pHpzc) of the adsorbent was determined by solid addition method (Helmy and Ferreiro 1976). 50 mL DDW was transferred to a series of conical flasks and the initial pH (pHi) of these solutions were roughly adjusted between 1 and 10 using either 0.1 M HCl or 0.1 M NaOH solution. The initial pH (pHi) of these solutions was accurately noted. 0.5 g adsorbent was then added to each flask and allowed to equilibrate for 24 h with intermittent manual shaking. The final pH (pHf) of the supernatant liquid was then noted. The difference between the initial pHi and pHf values were plotted against pHi, the point of intersection of the resulting curve with abscissa, at which (pHi − pHf) = 0, gave the pHpzc. To investigate the effect of electrolyte, same procedure was repeated using 0.5 M KNO3 solution.
Effect of time and initial concentration
Effect of time on the adsorption of Cd(II) was determined by analyzing the residual Cd(II) in the liquid after contact period from 5 to 240 min. Experiments were performed using batch process at room temperature. The amount of 0.5 g adsorbent was added to 50 mL solution of various initial concentrations of Cd(II) (50–80 mg L−1). Samples were withdrawn from conical flasks after specified time interval and analyzed for residual metal content.
Effect of electrolytes
The effect of various electrolytes such as CaCl2, NaHCO3, NaCl, MgCl2 as well as HCl on the adsorption of Cd(II) was investigated. In these experiments, 50 mL solution (50 mg L−1) of Cd(II) prepared in the above electrolytes was treated with 0.5 g of adsorbent. The amount of Cd(II) adsorbed in presence of these electrolytes were then determined as described earlier.
Breakthrough capacity
In this column experiment, 0.5 g adsorbent was taken in glass column with glass wool support. 500 mL of Cd(II) solution with 50 mg L−1 initial concentration (C0) was passed through the column with flow rate at 1 mL min−1. The effluent was collected in 50 mL fractions and the amount of Cd(II) (C) was determined in each fraction by AAS. The breakthrough capacity curve was obtained by plotting C/C0 versus volume of the effluent.
Results and discussion
Characterization of adsorbent
Adsorption of metal ions
The adsorption percentage of Pb(II), Cu(II), Ni(II), Cd(II) and Cr(VI) is shown in Fig. 1. The efficiency of adsorption was in order of Cd(II) > Pb(II) > Cu(II) > Cr(VI) > Ni(II). The % adsorption of Cd(II) was found to be maximum hence adsorption properties of Cd(II) were investigated in details.
Scanning Electron Microscopy (SEM)
The SEM micrographs of native and Cd(II) adsorbed bottlebrush seeds are shown in Fig. 2a, b. The morphology of the surface is slightly changed after Cd(II) adsorption. Adsorbed Cd(II) on the surface of the adsorbent can be seen clearly in the form of white patches.
Fourier transforms infrared spectroscopy (FTIR) analysis
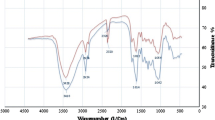
The pattern of adsorption of metals onto plant materials is attributable to the active groups and bonds present on the adsorbent surface (Krishnani et al. 2008). FTIR spectra of native and after adsorption of Cd(II) were recorded. The major functional groups present in native bottlebrush seed capsules responsible for Cd(II) adsorption were identified and are reported in Table 1. Peaks appearing in the FTIR spectra of native bottlebrush were assigned to various groups (Fig. 3). The peak at 3,421 cm−1 was assigned to the O–H group due to inter and intra molecular hydrogen bonding. The O–H stretching vibrations indicating the presence of free hydroxyl groups and bonded O–H bands of carboxylic acids (Gnanasambandam and Protor 2000). The band at 2,923 cm−1 indicates asymmetric C–H stretching vibration of aliphatic acids (Li et al. 2007). The peak at 2,856 cm−1 was the symmetric stretching vibration of CH2 due to C–H bonds of aliphatic acids (Guibaud et al. 2003). Peak observed at 1,736 cm−1 is the stretching vibration of C=O due to non-ionic carboxyl groups (–COOH, –COOCH3) and may be assigned to carboxylic acids or their esters (Li et al. 2007). At 1,649 cm−1 an asymmetric and symmetric vibration of ionic carboxyl groups (–COO−) has appeared. The peak at 1,372 cm−1 may be assigned to symmetric stretching of –COO− (Farinella et al. 2007). 1,036 cm−1 can be assigned to stretching vibration of C–OH of alcoholic groups and carboxylic acids (Guibaud et al. 2003). FTIR spectra of Cd(II) adsorbed bottlebrush showed that the peaks at 3,421, 2,923, 2,856, 1,736, 1,649, 1,372 and 1,036 cm−1 were shifted, respectively, to 3,416, 2,924, 2,860, 1,739, 1,643, 1,370 and 1,050 cm−1 due to Cd(II) interaction with these groups (Table 1).
Effect of contact time and initial concentration of Cd(II)
The mechanism of the metal uptake generally depends on the initial concentration of heavy metals in contact with the adsorbent. At low concentration the specific sites are responsible for the adsorption, while in case of increasing metal concentrations the specific sites are saturated and the adsorption sites are filled (Lehman and Harter 1984). The effect of initial concentration of Cd(II) on the extent of adsorption is shown in Fig. 4. It has been found that adsorption increased with time and attained maximum for all the concentrations. The adsorption capacities at 50, 60 and 80 mg L−1 initial Cd(II) concentrations were found to be 4.92, 5.4 and 6.0 mg g−1, respectively. This result might be due to increased driving force with increased Cd(II) concentration. The uptake of metal ions at any particular concentration increased with contact time. The rate was rapid but it slows down until it reaches equilibrium (Fig. 4). This is due to the fact that a large number of vacant surface sites are available for adsorption during the initial stage and with the increase of time the remaining vacant surface sites are difficult to be occupied due to repulsive forces between the solute molecules on the solid and bulk phases. However, with increase in initial Cd(II) concentration, the contact time needed to reach equilibrium was also increased. It has been observed that equilibrium was attained at 125, 30 and 10 min for 80, 60 and 50 mg L−1 of Cd(II) concentrations, respectively.
Effect of pH
The % adsorption of Cd(II) increased with increase in the initial pH (pHi) and attained maximum at pH 5. Adsorption was also influenced by equilibrium pH or final pH (pHf) of the solution. The mechanism of adsorption can be explained on the basis of initial pH (pHi), final (pHf), surface charge of the adsorbent and speciation of the metal. Adsorption of Cd(II) was 66 % at pH 2. When pHi was adjusted to 2 the final pH (pHf) increased to 3 indicating that some Cd(II) ions were adsorbed along with H+ ions resulted an increase in pHf. When pHi was increased further, final pH (pHf) increased slowly (4.0–4.8) and adsorption of Cd(II) increased to 95 %. Further increase in pHi (pH > 4) did not affect % adsorption. However, when initial pH (pHi) was adjusted to 6 pHf decreased to 4.7. This may be because at pH > 6 Cd(II) existed as Cd2+ ions (in large quantity) with the formation of Cd(OH)+ (small quantity) (Srivastava et al. 2006; Boparai et al. 2013) resulting a decrease in pHf. Therefore, it can be concluded that maximum amount of Cd(II) was adsorbed in the form of Cd2+ ions up to pH 8 with the formation of small quantity of Cd(OH)+. It has also been observed that adsorption of Cd(II) was suppressed in presence of high concentration of electrolyte (0.5 M KNO3). Adsorption of Cd(II) was less at all the studied PHS in presence of KNO3 (Fig. 5).This may be due to decrease in Cd(II) activity at high concentration of electrolyte. The decrease in the activity of Cd(II) ions is due to the formation of soluble and stable complexes with anions which are not easily adsorbed (Malamis and Katsou 2013). The point of zero charge pHpzc (Babic et al. 1999) determined in DDW and 0.5 M KNO3 is shown in Fig. 6. The point of intersection where ∆pH = 0 was shifted to lower pH in presence of KNO3 indicated that H+ ions were displaced by K+ ions (due to their large excess) and hence responsible for the suppression of Cd(II) adsorption. However, second charge reversal observed at pH 1 (calculated by extrapolation of these curves) where influence of K+ ions was negligible and so the % adsorption of Cd(II) was affected least at pH 2 in presence of KNO3.
Adsorption isotherms
To optimize the design of adsorption system for the removal of Cd(II) from aqueous solution, it is important to explain the relationship between adsorbed metal ion per unit weight of adsorbent (qe) and residual concentration of metal ion in solution (Ce) at equilibrium. The analysis of the adsorption isotherm data by fitting them to different adsorption isotherm models is an important step to find the suitable adsorption isotherm model that can be used for design purposes. Experimental data were fitted in the Langmuir, Freundlich, Temkin and D–R models at different temperatures. The fitting procedure was performed using R-software version 2.10.1 (2009-12-14). To evaluate the fitness of the data, determination coefficient (R2), error analysis and chi-square test (χ2) were evaluated between experimental and calculated data from each model.
According to Langmuir model (Langmuir 1916) the adsorption occurs on a homogenous surface forming monolayer of adsorbate with constant heat of adsorption for all sites without interaction between adsorbed molecules (Kalavathy and Miranda 2010). The linear form of Langmuir model may be given as.
where Ce is the equilibrium concentration of Cd(II) in the solution (mg L−1), qe is the amount of Cd(II) adsorbed per unit weight of adsorbent (mg g−1), qm is the amount of Cd(II) required to form monolayer (mg g−1) and b is a constant related to energy of adsorption (L mg−1) which represents enthalpy of adsorption and should vary with temperature. The values of b and qm were calculated from the slope and intercept of the linear plots of 1/qe versus 1/Ce at different temperatures. The data obtained from this model indicated its applicability at different temperature (30–50 °C) but data were fitted best at higher temperatures (40 and 50 °C) as indicated by high determination coefficient (R2), RSE and least χ2 values (Table 2). The values of qm and b increased with increasing temperature indicating higher heat of adsorption with increasing temperature and confirming the endothermic nature of the adsorption. Table 3 lists a comparison of maximum monolayer adsorption capacity (qm) of Cd(II) on various adsorbents (Singh et al. 2010; Al-Anber and Matouq 2008; Ghodbane et al. 2008; Gupta and Rastogi 2008; Iqbal et al. 2009; Zheng et al. 2010; Naiya et al. 2009; Zhang et al. 2012; Gilbert et al. 2011; Rao and Kashifuddin 2012a, b). Bottlebrush was found to have a relatively larger adsorption capacity indicating that it could be considered a promising new material for the removal of Cd(II) from aqueous solutions.
The Freundlich model (Freundlich 1906) is an empirical equation based on the adsorption of adsorbate onto heterogeneous surface. The linear form of Freundlich model can be represented as.
where Ce is the equilibrium concentration in mg L−1, Kf is the Freundlich constant which indicates the relative adsorption capacity of the adsorbent related to bonding energy and n is the heterogeneity factor representing the deviation from linearity of adsorption and is also known as Freundlich coefficient. A plot of log qe versus log Ce should generate straight line and values of 1/n and Kf can be calculated from the slope and intercept. The data obtained from this model indicated that the values of Kf and n increased with the increase in temperature from 30 to 50 °C. Freundlich model was best obeyed at 50 °C because of high R2 and least χ2 values (Table 2). The n values between 0.0 and 1.0 indicated favourable adsorption (Arfaoui et al. 2008). Table 3 also shows Freundlich isotherm parameters of various adsorbents and it was comparable and better than those reported in literature (Tan and Xiao 2009; Rao and Khan 2009; Xie et al. 2011; Sharma et al. 2009; Etci et al. 2010; Al Othman et al. 2011; Boparai et al. 2011).
Temkin isotherm (Temkin and Pyzhev 1940) assumed indirect adsorbate/adsorbent interactions on adsorption isotherms and explains that heat of adsorption of all the molecules on the adsorbent surface decreases linearly rather than logarithmic as implied in Freundlich isotherm. The linearized form of Temkin equation can be represented as
where (RT/b) = B, R is universal gas constant, T is absolute temperature and b is Temkin isotherm constant. A (L mg−1) is equilibrium binding constant corresponding to maximum binding energy and B (J mol−1) is another constant related to heat of adsorption. The values of B and A were calculated from the slope and intercept of the plot of qe versus ln Ce and are reported in Table 2. Increased values of A with increasing temperature indicated that process is favourable at high temperature.
Dubinin–Redushkeuich (D–R) isotherm (Dubinin and Radushkevich 1947) does not assume a homogenous surface or a constant sorption potential (Gonzalez et al. 2006). The Dubinin–Radushkevich model was chosen to estimate mean free energy of adsorption. The linearized form of this equation is represented as
where ε is the Polyani potential, qm is the monolayer capacity (mol g−1), Ce is the equilibrium concentration (mol L−1). The Polyani potential (ε) and mean free energy of adsorption (E, kJ mol−1) can be calculated from the equations. β is the D–R model constant (mol2 kJ−1) which can be obtained from the slope of the plot of ln qe verses ε2. The values of E lie between 8 and 16 kJ mol−1 and depicted that adsorption process was chemical in nature (Helfferich 1962). The positive value of energy (E) of adsorption indicated that the adsorption process is endothermic and confirmed that higher solution temperature will favour the adsorption process.
It can be concluded from the Table 2, that the above models were well fitted at higher temperature (50 °C) as indicated by high determination coefficient (R2), low values of RSE, and least Chi-square (χ2) test values. The p value for all the models at 30, 40 and 50 °C were <0.05 (p < 0.05). The Langmuir, Freundlich and D–R adsorption isotherm models fits the experimental data (at higher temperatures) better than Temkin isotherm model.
Thermodynamic study
The effect of temperature on the adsorption of Cd(II) was studied at temperature ranging from 30 to 50 °C. Thermodynamic parameters such as standard free energy change (ΔG°), standard enthalpy change (ΔH°) and standard entropy change (ΔS°) were calculated using the following relations (Liu 2009)
where Kc is the distribution constant, CAd and Ce are equilibrium concentrations of Cd(II) on the adsorbent and in the solution, respectively. According to the IUPAC (1947) the distribution constant is defined as the ratio of the concentration of a substance in a single definite form in the extract to its concentration in the same form in the other phase at equilibrium. The Gibbs energy change (ΔG°) indicates the degree of spontaneity of an adsorption process, and a higher negative value reflects a more energetically favourable adsorption. According to thermodynamic law, ΔG° of adsorption is calculated as follows
where Kc is the thermodynamic equilibrium constant without units, T is the absolute temperature in Kelvin and R is the gas constant. The values of ΔH° and ΔS° were calculated from the following Van’t Hoff equation
A plot of ln Kc versus 1/T gives straight line and ΔH° and ΔS° were calculated from the slope and intercept. These thermodynamic parameters can offer insight into the type and mechanism of an adsorption process (Reported in Table 4). Values of free energy change ΔG° are negative confirming that adsorption of Cd(II) is spontaneous and thermodynamically favourable since ΔG° became more negative with increase in temperature, indicating high driving force and hence resulting in higher adsorption capacity at higher temperature. The positive value of ΔH° indicated endothermic adsorption process. A little but positive value of ΔS° in the temperature range 30–50 °C suggested increased randomness at the solid-solution interface because some water molecules were dislodged during adsorption of Cd(II) (Narmasivayam and Ranganathan 1995).
Adsorption kinetics
The rate constants were calculated using pseudo-first-order (Lagergren 1898) and pseudo-second-order kinetics models. The first-order rate expression is given below
where qe is the amount of Cd(II) adsorbed per unit weight of adsorbent at equilibrium or adsorption capacity (mg g−1), qt is the amount of Cd(II) adsorbed per unit weight of adsorbent at any given time t, K1 is the rate constant for pseudo-first-order model. The values of K1 and qe were calculated from slope and intercept of the linear plot of log (qe − qt) versus t at various concentrations. A plot of log (qe − qt) versus t gave straight lines confirming the applicability of the pseudo-first-order rate equation.
The pseudo-second-order rate expression is used to describe chemisorption involving valence forces through the sharing or exchange of electrons between the adsorbent and adsorbate as covalent forces, and ion exchange (Ho and Mckay 1998). The pseudo-second-order kinetic rate equation is given as
where h is the initial rate of adsorption (h = K2q 2e ) and K2 is the rate constant of pseudo-second-order adsorption (g mg−1 min−1). The values of K2 and qe were calculated from the intercept and slope of the linear plots of t/qt versus t at various Cd(II) concentrations. Straight line plots of t/qt versus t indicated the applicability of pseudo-second-order model (Fig. 7).
Table 5 provides data of pseudo-first-order rate constants K1, pseudo-second-order rate constants K2, initial adsorption rate (h), R2, calculated equilibrium adsorption capacity qe (cal) and experimental equilibrium adsorption capacity qe (exp) at different initial Cd(II) concentrations. It was found that qe (cal) values from pseudo-first-order model differed appreciably from the experimental values showing that system did not follow pseudo-first-order model. In pseudo-second-order kinetic model the values of qe (cal) were very close to qe (exp) values at various initial Cd(II) concentrations as compared to pseudo-first-order model indicating that pseudo-second model was better obeyed. The data also shows that the values of determination coefficient (R2) for pseudo-first-order model (Table 5) were lower as compared to pseudo-second-order kinetic model at different initial concentration values.
Breakthrough capacity
Breakthrough curve is the most effective column process making the optimum use of the concentration gradient between the solute adsorbed by the adsorbent and that remaining in the solution. The column is operational until the metal ions in the effluent start appearing and for practical purposes the working life of the column is over called breakthrough point. This is important in process design because it directly affects the feasibility and economics of the process (Gupta et al. 2001). Figure 8 showed that 150 mL of Cd(II) solution could be passed through column without detecting Cd(II) in the effluent. The breakthrough capacity was found to be 15 mg g−1.
Effect of electrolytes
Cd(II)-contaminated water also contains several other ions that may influence the adsorption process. This study evaluated the behaviour of Cd(II) adsorption in the presence of 0.01–0.1 M salt solution of various electrolytes like chloride, carbonate, sulphate, independently at an initial Cd(II) concentration of 50 mg L−1. The effect of various electrolytes on the adsorption of Cd(II) is reported in Table 6. Adsorption of Cd(II) was decreased with increase in concentration of electrolytes like CaCl2, NaCl, Na2SO4, MgCl2, HCl. This could be attributed to increased competition for adsorption sites between Cd(II) ions and electrolyte ions as well as decreased activity of Cd(II) ions (Malamis and Katsou 2013). The influence of these electrolytes and their effect in toxic pollutants removal using natural materials has been observed in recent past (Pehlivan et al. 2011; Gladysz-Plaska et al. 2012; Altun and Pehlivan 2012; Pang et al. 2011; Jing et al. 2011; Rao and Kashifuddin 2012a, b; Wang et al. 2011; Chen et al. 2010; Akafi et al. 2011). Many of these studies also showed a decrease in the adsorption of metal ions in the presence of electrolytes.
However, adsorption of Cd(II) increased with increasing NaHCO3 concentrations which may be due to increase in final pH (pHf) of the NaHCO3 solution with increased concentration (pH 7.8–8.8) as reported in Table 6.
Conclusions
Bottlebrush seeds showed excellent potential for Cd(II) removal from aqueous solution. Equilibrium time was concentration dependent. The experimental results showed that the adsorbent always had a higher capability to adsorb Cd(II) ions and the maximum adsorption could be possible from the aqueous solution at pH 4. Langmuir, Freundlich, Temkin and D–R isotherm were best obeyed at higher temperatures. Adsorption of Cd(II) was affected by increasing concentration of various electrolytes. Kinetic data showed the better applicability of pseudo-second-order model confirming that adsorption of Cd(II) was chemisorption in nature. Thermodynamic results indicated the adsorption of Cd(II) was spontaneous and endothermic. Column experiments showed that the breakthrough began at 150 mL.
References
Adie DB, Okuofu CA, Osakwe C (2012) Isothermal and batch adsorption studies of the use of Borassus aethiopium and Cocos nucifera for wastewater treatment. Am Inter J Contemp Res 2:119–130
Akafi MM, Reich TJ, Koretsky CM (2011) Assessing Cd Co, Cu, Ni, and Pb sorption on montmorillonite using surface complexation models. Appl Geochem 26:154–157
Almasi A, Omidi M, Khodadadian M, Khamutian R, Gholivand MB (2012) Lead(II) and cadmium(II) removal from aqueous solution using processed Walnut shell: kinetic and equilibrium study. Toxic Environ Chem 94:660–671
Al-Anber ZA, Matouq MAD (2008) Batch adsorption of cadmium ions from aqueous solution by means of olive cake. J Hazard Mater 151:194–201
Altun T, Pehlivan E (2012) Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem 132:693–700
Al Othman ZA, Hashem A, Habila MA (2011) Kinetic, equilibrium and thermodynamic studies of cadmium(II) adsorption by modified agricultural wastes. Molecules 16:10443–10456
Arfaoui S, Frini-Srasra N, Srasra E (2008) Modelling of the adsorption of the chromium ion by modified clays. Desalination 222:474–481
Babic BM, Milonjic SK, Polovina MJ, Kaludierovic BV (1999) Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon 37:477–481
Bayo J, Esteban G, Castillo J (2012) the use of native and protonated grapefruit biomass (Citrus paradisi L.) for cadmium(II) biosorption: equilibrium and kinetic modelling. Environ Tech 33:761–772
Benoff S, Jacob A, Hurley IR (2000) Male Infertility and environmental exposure to lead and cadmium. Hum Reprod Update 6:107–121
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zero valent iron particles. J Hazard Mater 186:458–465
Boparai HK, Joseph M, O’Carroll DM (2013) Cadmium (Cd2+) removal by nano zerovalent iron: Surface analysis, effects of solution chemistry and surface complexation modelling. Environ Sci Poll Res 20:6210–6221
Bouziane L, Bendebane F, Ismail F, Delimi R (2012) Removal of zinc and cadmium from an aqueous solution using sawdust as a low-cost adsorbent: application of Plackett-Burman design. Desalin Water Treat 49:189–199
Cazon JP, Bernardelli C, Viera M, Donati E, Guibal E (2012) Zinc and cadmium biosorption by untreated and calcium-treated Macrocystis pyrifera in a batch system. Biores Technol 116:195–203
Chao H-P, Chang C-C (2012) Adsorption of copper(II), cadmium(II), nickel(II) and lead(II) from aqueous solution using biosorbents. Adsorption 18:395–401
Chen L, Lu L, Shao W, Luo F (2011) Kinetics and equilibria of Cd(II) adsorption onto a chemically modified Lawny Grass with H [BTMPP]. J Chem Eng Data 56:1059–1068
Chen N, Zhang Z, Feng C, Sugiura N, Li M, Chen R (2010) Fluoride removal from water by granular ceramic adsorption. J Coll Inter Sci 348:579–584
Chen Y, Ding L, Jinxia N (2012) Isotherm and thermodynamic studies of the biosorption of lead, cadmium and copper from aqueous solutions by rice bran. Desalin Water Treat 44:168–173
Cribb AB, Cribb JW (1976) Wild food in Australia, a very good pocket guide, Fontana, ISBN 0-00-634436-4
Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium(II) by unmodified rice straw. Biores Technol 114:20–25
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Phys Chem Sec C 55:331–337
Etci O, Bektas N, Oncel MS (2010) Single and binary adsorption of lead and cadmium ions from aqueous solution using the clay mineral beidellite. Environ Earth Sci 61:231–240
Farinella NV, Matos GD, Arruda MAZ (2007) Grape bagasse as a potential biosorbent of metals in effluent treatments. Biores Technol 98:1940–1946
Fourest E, Roux J (1992) Heavy metal biosorption by fungal mycelial by products: mechanism and influence of pH. Appl Microbiol Biotechnol 37:399–403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Friberg L, Nordberg GF, Vouk VB (1986) Handbook of the toxicology of metals, vol II. Elsevier, New York, pp 130–184
Genders R (1977) Scented Flora of the world an excellent, comprehensive book on scented plants. Hale, R, Pennsylvania State University, University Park
Gilman, EF, Watson, DG (1993) Callistemon viminalis weeping bottlebrush, fact sheet ST-111. A series of the Environmental Horticulture Department Institute of Food and Agricultural Sciences
Ghodbane I, Nouri L, Hamdaoui O, Chiha M (2008) Kinetic and equilibrium study for the sorption of cadmium(II) ions from aqueous phase by eucalyptus bark. J Hazard Mater 152:148–158
Gilbert UA, Emmanuel IU, Adebanjo AA, Olalere GA (2011) Biosorptive removal of Pb2+and Cd2+ onto novel biosorbent: defatted Carica papaya seeds. Biomass Bioeng 35:2517–2525
Gladysz-Plaska A, Majdan M, Pikus S, Sternik D (2012) Simultaneous adsorption of chromium(VI) and phenol on natural red clay modified by HDTMA. Chem Eng J 179:140–150
Gnanasambandam R, Protor A (2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 68:327–332
Gonzalez JR, Videa JRP, Rodriguez E, Delgado M, Gardea-Torresdey JL (2006) Potential of Agave lechuguilla biomass for Cr(III) removal from aqueous solutions: thermodynamic studies. Biores Technol 97:178–182
Guibaud G, Tixier N, Bouju A, Baudu M (2003) Relation between extracellular Polymer’s composition and its ability to complex Cd, Cu and Pb. Chemosphere 52:1701–1710
Gupta VK, Rastogi A (2008) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass. Oedogonium sp. J Hazard Mater 153:759–766
Gupta VK, Shrivastava AK, Jain N (2001) Biosorption of chromium(VI) from aqueous solutions by green algae Spirogyra species. Water Res 35:4079–4085
Helfferich F (1962) Ion exchange. McGraw-Hill, New York 166
Helmy AK, Ferreiro EA (1976) The aluminium oxide aqueous interface and point of zero charge. Zeitsch fur Physik Chem 257:881–892
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Proc Saf Environ Prot 6:332–340
Hydari S, Sharififard H, Nabavinia M, Parvizi MR (2012) A comparative investigation on removal performances of commercial activated carbon, chitosan biosorbent and chitosan/activated carbon composite for cadmium. Chem Eng J 193–194:276–282
IUPAC (1947) Compendium of chemical terminology, 2nd edn. Blackwell Science, Oxford, U.K
Iqbal M, Saeed A, Zafar SI (2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ionexchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J Hazard Mater 164:161–171
Jing X, Cao Y, Zhang X, Wang D, Wu X, Xu H (2011) Biosorption of Cr(VI) from simulated wastewater using a cationic surfactant modified spent mushroom. Desalination 269:120–127
Kalavathy MH, Miranda LR (2010) A solid phase extractant for the removal of copper, nickel and zinc from aqueous solutions. Chem Eng J 158:188–199
Kenduzler E, Turker AR (2005) Optimization of a new resin, Amberlyst 36, as a solid phase extractor and determination of copper(II) in drinking water and tea samples by flame atomic absorption spectrometry. J Sep Sci 28:2344–2349
Krishnani KK, Xiaoguang MX, Christodoulatos C, Boddu VM (2008) Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 153:1222–1234
Kumar PS, Gayathri R, Senthamarai C, Priyadarshini M, Fernando PSA, Srinath R, Kumar VV (2012a) Removal of cadmium(II) from aqueous solution by agricultural waste cashew nut shell. Korean J Chem Eng 29:1752–1760
Kumar PS, Ramalingam S, Vijayakumar R, Abhinaya Kirupha SD, Murugesan A, Sivanesan S (2012b) Adsorption of metal ions onto the chemically modified agricultural waste. Clean Soil Air Water 40:188–197
Lagergren S (1898) About the theory of so called adsorption of soluble substances. Kun Sven Vetenskap adem Handl 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Lehman RG, Harter RD (1984) Assessment of copper–soil bond strength by desorption kinetics. Soil Sci Soc Am J 48:769–772
Leyva-Ramos R, Landin-Rodriguez LE, Leyva-Ramos S, Medellin-Castillo NA (2012) Modification of corncob with citric acid to enhance its capacity for adsorbing cadmium(II) from water solution. Chem Eng J 180:113–120
Li FT, Yang H, Zhao Y, Xu R (2007) Novel modified pectin for heavy metal adsorption. Chin Chem Lett 18:325–328
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54:1981–1985
Marques P, Rosa MF, Pinheiro HM (2000) pH effects on the removal of Cu2+, Cd2+ and Pb2+ from aqueous solution by waste brewery biomass. Bioproc Eng 23:135–141
Masoudi H, Akbarzadeh S, Barghamadi M (2012) Removal of chromium(VI) and cadmium from aqueous solution using orange peel as a cheap adsorbent. J App Sci Environ Sanit 7:269–273
Merrikhpour H, Jalali M (2012) Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Technol Environ Policy J 14:845–855
Mohapatra M, Anand S (2007) Studies on sorption of Cd(II) on Tata chromite mine overburden. J Hazard Mater 148:553–559
Malamis S, Katsou E (2013) A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms. J Hazard Mater 252–253:428–461
Naiya TK, Chowdhery P, Bhattacharya AK, Das SK (2009) Saw dust and neem bark as low cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chem Eng J 148:68–79
Narmasivayam C, Ranganathan K (1995) Removal of Cd(II) from wastewater by adsorption on waste Fe(III)/Cr(III) hydroxide. Water Res 29:1737–1744
Omorogie MO, Babalola JOE, Unuabonah I, Gong JR (2012) Kinetics and thermodynamics of heavy metal ions sequestration onto novel Nauclea diderrichii seed biomass. Biores Technol 118:576–579
Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X, Li Z, Zhang J, Liu Z, Xiong Y (2011) Preparation and application of stability enhanced magnetic nanoparticles for rapid removal of Cr(VI). Chem Eng J 175:222–227
Pehlivan E, Kahraman H, Pehlivan E (2011) Sorption equilibrium of Cr(VI) ions on oak wood charcoal (Carbo Ligni) and charcoal ash as low-cost adsorbents. Fuel Process Technol 92:65–70
Prakash N, Sudha PN, Renganathan NG (2012) Copper and cadmium removal from synthetic industrial wastewater using chitosan and nylon 6. Environ Sci Poll Res 19:2930–2941
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2012) Removal of cadmium(II) from aqueous solution by adsorption using meranti wood. Wood Sci Technol 46:221–241
Rao RAK, Kashifuddin M (2012a) Adsorption properties of coriander seed powder (Coriandrum sativum): extraction and preconcentration of Pb(II), Cu(II) and Zn(II) from aqueous solution. Adsorpt Sci Technol 30:127–146
Rao, RAK, Kashifuddin, M (2012) Adsorption studies of Cd(II) on Ball Clay: comparison with other natural clays. Arab J Chem. doi:10.1016/j.arabjc.2012.01.010
Rao RAK, Khan MA (2009) Biosorption of bivalent metal ions from aqueous solution by an agricultural waste: kinetics, thermodynamics and environmental effects. Coll Surf A Physicochem Eng Asp 332:121–128
Rao RAK, Rehman F, Kashifuddin M (2012) Removal of Cr(VI) from electroplating wastewater using fruit peel of leechi (Litchi chinensis). Desalin Water Treat 49:136–146
Rao RAK, Rehman F (2012) Use of Polyalthia longifolia seeds (seeds of Indian Mast Tree) as adsorbent for the removal of Cd(II) from aqueous solution. J Dispers Sci Technol 33:472–481
Ruiz-Manriquez A, Magana PI, Lopez V, Guzman R (1998) Biosorption of Cu by Thiobacillus ferrooxidans. Bioproc Eng 18:113–118
Satarug S, Haswell-Elkins MR, Moore MR (2000) Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br J Nutr 84:791–802
Srivastava VC, Indra Deo Mall ID, Misra M (2006) Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse ash. Chem Eng J 117:79–91
Sharma N, Kaur K, Kaur S (2009) Kinetic and equilibrium studies on the removal of Cd2+ ions from water using polyacrylamide grafted rice (Oryza sativa) husk and (Tectona grandis) saw dust. J Hazard Mater 163:1338–1344
Singh R, Chadetrik R, Kumar R, Bishnoi K, Bhatia D, Kumar A, Bishnoi NR, Singh N (2010) Biosorption optimization of lead(II), cadmium(II) and copper(II) using response surface methodology and applicability in isotherms and thermodynamics modelling. J Hazard Mater 174:623–634
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2012) Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ Sci Poll Res. doi:10.1007/s11356-012-1208-2
Tan G, Xiao D (2009) Adsorption of cadmium ion from aqueous solution by ground wheat stems. J Hazard Mater 164:1359–1363
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim Sin 12:327–356
Wang Q, Chang X, Li D, Hu Z, Li R, He Q (2011) Adsorption of chromium(III), mercury(II) and lead(II) ions onto 4-aminoantipyrine immobilized bentonite. J Hazard Mater 186:1076–1081
Waseem S, Din MI, Nasir S, Rasool A (2012). Evaluation of Acacia nilotica as a non-conventional low cost biosorbent for the elimination of Pb(II) and Cd(II) ions from aqueous solutions. Arab J Chem. doi:10.1016/j.arabjc.2012.03.020
Xie G, Shang X, Liu R, Hu J, Liao S (2011) Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohyd Polym 84:430–438
Zamani AA, Shokri R, Yaftian MR, Parizanganeh AH (2013) Adsorption of lead, zinc and cadmium ions from contaminated water onto Peganum harmala seeds as biosorbent. Int J Environ Sci Technol 10:93–102
Zhang D, Zeng X, Ma P, He H, Falandysz J (2012) The sorption of Cd(II) from aqueous solutions by fixed Lentinus edodes mushroom flesh particles. Desalin Water Treat 46:21–31
Zheng L, Dang Z, Yi X, Zhang H (2010) Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk. J Hazard Mater 176:650–656
Acknowledgments
Authors are thankful to the Chairman, Department of Applied Chemistry, Z.H. College of Engineering and Technology, A.M.U. for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rao, R.A.K., Kashifuddin, M. Kinetics and isotherm studies of Cd(II) adsorption from aqueous solution utilizing seeds of bottlebrush plant (Callistemon chisholmii). Appl Water Sci 4, 371–383 (2014). https://doi.org/10.1007/s13201-014-0153-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0153-2