Summary
Prompted by concurrent completion of the latest plant atlas for Britain and Ireland, the orchid flora of these islands is reviewed in detail, focusing on 21st century progress in both systematics research and formal conservation categorisation under IUCN criteria. DNA-based phylogenies consistently circumscribe monophyletic groups that constitute obvious genera, though relationships among those genera remain less certain and resistance to phylogenetic classification persists. Comparison of species circumscription studies in critical groups such as Dactylorhiza, Gymnadenia, Platanthera, Epipactis and Orchis shows that most of the recent progress has been achieved using DNA-based techniques, which have also greatly increased understanding of associated mycorrhizae. Most morphological studies remain disappointingly traditional, squandering opportunities for morphometric studies that identify discontinuities between species, allow comparison of rates of morphological and molecular evolution, and underpin accurate quantitative descriptions designed to aid identification. Possibly only one of the 53 species recognised as native is still viewed as endemic. Conventional views on the significance of both pollinator specificity and quantitative seed output as the preferred measure of fitness are questioned, and mycorrhizae are explored as possibly under-rated ecological filters. Comparison of species distributions in successive Atlases suggests diverse patterns of change that reflect a combination of habitat modification, climate change and, in a few cases, improved identification skills. Ecologically tolerant lowland species such as Ophrys apifera and Dactylorhiza praetermissa are migrating northward rapidly, while the genus Serapias appears to be mounting a cross-Channel invasion. Most formal Red-Listing categorisations, and the underlying IUCN criteria, are supported for decisions made within the British Isles, but unfortunately, they have translated poorly into the equivalent European and Global lists. Within Britain, the Data Deficient category has been employed too frequently and illogically; ironically, it is sometimes used to penalise species for having been the subjects of serious research. Far from being "complete", the present consensus view on the taxonomy and evolution of the glacially impoverished British and Irish flora is judged both parochial and complacent. Reinvigorated UK-based research programmes that integrate quantitative morphological and molecular studies of British and Irish natives in a European context are urgently needed.
Similar content being viewed by others
Introduction
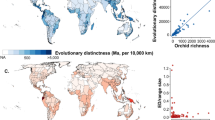
Approximately every two decades, the Botanical Society of Britain and Ireland and Biological Records Centre collaborate to produce a hectad-scale plant atlas summarising the current state of the British and Irish vascular flora (Perring & Walters 1962; Perring & Walters 1982 [partial revision]; Preston et al. 2002; Stroh et al. 2022). Field botany and, at least in some genera, taxonomic research are stimulated by these projects prior to publication. Once released, each atlas then usefully catalyses studies of biogeography and ecology, and leads to revised national conservation priorities. The publication of a new plant atlas, on this occasion Atlas 2020 (Stroh et al. 2022), therefore constitutes the ideal point to review these properties for particular plant families in Britain and Ireland. Here, the reviewed family is the Orchidaceae, which encompasses 53 putatively native species. Orchids not only represent approximately 4% of our native vascular flora but also include a much higher percentage of charismatic conservation "pin-ups".
I last published an overview of the orchid flora of the British Isles 16 years ago (Bateman 2006), following the release of Atlas 2000 (Preston et al. 2002) and in collusion with the authors of the consequent update of the Red List for British Vascular Plants (Cheffings et al. 2005). These works were also important in providing early indications of range expansions and contractions (Kull & Hutchings 2006), and in pre-empting changes in the third edition of Stace's (2010) definitive British and Irish flora. Much of my 2006 text was devoted to critically appraising insights gained during the previous decade through DNA-based molecular studies. Sanger sequencing of a very few candidate gene regions had generated evolutionary trees (phylogenies) that often challenged previous morphology-based classifications and, when combined with morphometric methods, promised to revolutionise taxonomic circumscription at the population level. Today, we see Sanger sequencing being progressively replaced by high-throughput (sometimes termed 'next-generation' or 'post-Sanger') sequencing, yielding orders of magnitude more data from each studied plant (e.g. Olson et al. 2016; Eserman et al. 2021). However, advances of the last 16 years have not dulled the ensuing taxonomic debates, which bizarrely still often concern the degree of relevance of molecular data, not just the best means of employing them in a taxonomic context.
Taxonomic circumscription feeds into identification guides (notably the fourth edition of Stace 2019) that underpin field surveys that in turn, through computerised databases, generate geographic distributions. The resulting quantified distributions dictate both ecological interpretation and conservation assessment — crucial areas that have become even more inextricably interlinked following widespread recognition of the impact of accelerating climate change (e.g. Hodkinson et al. 2011; Keppel et al. 2012; Groom 2013; Pettorelli et al. 2019). Each such cycle is a stepwise process that inevitably incurs a long inherent lag phase, highlighting the need to accelerate efforts to feed recent taxonomic insights directly into the mapping process.
I have divided this text into two main parts. Part 1 reviews increases in knowledge of particular genera of British and Irish orchids gained during the 21st century. Recent research has predictably focused on the more 'critical' genera — those that are more taxonomically diverse, controversial and/or especially prone to hybridisation: Dactylorhiza, Gymnadenia, Platanthera (together comprising the "digitate-tubered clade" sensu Bateman et al. 2018a), Orchis (subgenus Orchis) and Epipactis (subgenus Epipactis). Special mention is also made of the genus Serapias, in the light of what appears to be its sudden predilection for migration northward from the Continent (for the purposes of this review, the Channel Islands are regarded as belonging phytogeographically to Continental Europe). Part 2 outlines any potentially significant recent changes in species distribution and/or frequency, before suggesting some revisions to current IUCN conservation criteria and priorities, and flagging areas most in need of future research. I conclude this "state of the union address for orchids" by advocating a less parochial and more scientific approach to studying the British and Irish flora.
PART 1: SYSTEMATICS
Phylogenetics
Figure 2 of Bateman (2006) summarised molecular phylogenetic knowledge of British and Irish orchids as it existed in 2005. Here, I have updated that phylogeny as the present Fig. 1, after reviewing a further 16 years of phylogenetic research. Circled numbers on this composite phylogeny represent one or more individual studies focused on that particular portion of the orchid family tree (summarised in Table 1). It is symptomatic of the increasing molecular dominance of phylogeny reconstruction that, of the 26 studies cited in Table 1, only Bateman et al. (2018a) included an attempt to reconstruct phylogeny using morphological data. Morphological data have come to be regarded (wrongly, in my view) as being too prone to homoplasy and too time-consuming to gather and convert into an analysable matrix.
Grafted aggregate phylogeny of all bona fide British and Irish orchid species, based on 29 more focused published DNA-based analyses. Thick branches denote the more reliable relationships, and arrows denote apparent ancestor–descendant relationships. Obligately mycoheterotrophic species are asterisked, extirpated species are placed in parentheses, and one species confined to the Channel Islands (politically British but biogeographically French) is placed in brackets. Circled numbers indicate sources of cladograms contributing to that component of the overall topology, as detailed in Table 1. Symbols indicate nectar status: circle = nectar absent, annulus = modest amount of nectar secreted, spot = substantial reservoir maintained in spur. (Compare with fig. 2 of Bateman 2006.)
Many factors interact to determine the quality of a molecular tree constructed from the four nucleotides that constitute DNA. Gene sampling decisions focus on which of the three plant genomes to investigate and what proportion of each genome to sequence. Plant sampling decisions should consider number of plants per population, number of populations per putative species, and number of species under consideration (for both ingroup and outgroup), and should pit these factors against cost per sampled plant for the chosen data-generating technique(s). Less often discussed are the taxonomic and geographic completeness of sampling: whether to select representative species from across a wide range of taxa, or instead attempt to be comprehensive within a narrower taxonomic spectrum, and whether to constrain sampling geographically or attempt to encompass the entire geographic spread of the relevant group. The final set of obligatory decisions relate to data analysis; how best to "clean" the data computationally (a highly complex challenge with relatively recent high-throughput sequencing techniques), whether to combine data for different genes or analyse them separately, and which mathematical algorithms to select for building the resulting tree(s).
The last 16 years have seen gradual increases in the average number of plants analysed per study and the number of genes characterised through single-gene Sanger sequencing. Sample sets have gradually expanded as analytical costs have progressively decreased. Nonetheless, Table 1 demonstrates that the basic approach to phylogeny reconstruction did not change during most of the period under review; in most published studies, Sanger sequencing was used to generate data for ribotypes (nrITS) plus typically between one and five plastid regions. Thus, the story from 2005 to 2018 is one of increasing numbers of phylogenies that relied on broadly similar levels of underlying data. However, differences in choices of plants analysed and tree-building methods have often led to contradictory — in some cases, strongly contradictory — topologies that inferred different relationships and thus implied contrasting evolutionary patterns. These uncertainties inevitably impacted on orchid classification, encouraging detractors to disparage the entire discipline.
Only during the last three years have phylogenies been published that employ high-throughput sequencing techniques that are capable of revealing genetic differences that are orders of magnitude greater in number. RAD-seq is a technique that yields vast amounts of nuclear data at genus level and below, and thus far has been applied to Ophrys (Bateman et al. 2018b), Gymnadenia s.l. (Brandrud et al. 2019), Dactylorhiza (Brandrud et al. 2020) and Neotinea (Trávníček et al. 2021). In contrast, genome skimming — a technique applied to the Ophrys sphegodes clade by Bateman et al. (2021a) — yields almost complete plastomes, together with a selection of multi-copy nuclear regions that includes the ever-popular nrITS region. Toward the higher end of the taxonomic scale, broad-brush phylogenies that sample tribes sparsely in order to encompass the entire orchid family have recently been generated for 78 plastid genes (i.e. almost complete plastomes: Serna-Sánchez et al. 2021) and for 294 nuclear genes using target capture methods (Peréz-Escobar et al. 2021). At suprageneric level, the resulting taxonomic changes affect only the perennially troublesome obligate mycoheterotrophs; specifically, these studies tentatively suggest transfers of Corallorhiza trifida from tribe Calypsoeae to tribe Malaxidae, and of Epipogium aphyllum from tribe Gastrodieae to tribe Nervilieae.
High-throughout techniques have demonstrated the dangers of building trees using small numbers of genes. For example, a transcriptome study of just single representatives of four species of the genus Gymnadenia s.l. showed that six of 15 theoretically possible topologies each had the support of at least 9% of the 478 gene families sequenced, and that the two best-supported topologies (33% vs 18%) were the precise converse of each other (Pineiro-Fernández et al. 2019). Such equivocal results imply unstable rooting of the trees, and emphasise the importance of including multiple outgroups in such analyses. NGS techniques have already rendered obsolete the previously popular AFLP approach to gene fragmentation, and they are now causing phylogenetic studies based on Sanger sequencing (i.e. the great majority of those listed in Table 1) to appear increasingly passé. Importantly, the de facto downgrading of single-gene phylogenetics also undermines the concept of genetic "bar-coding" — an approach that earlier this century was sometimes portrayed as the potential salvation of plant identification (e.g. Hollingsworth et al. 2009; Bateman 2016), but that similarly depends on very limited data derived from between one and three gene regions.
It is crucial to appreciate that a parochial phylogeny based exclusively on the analysis of orchid species occurring in the British Isles would undoubtedly yield an incorrect topology, even if it was based on NGS data. Our (understandably rather modest) orchid flora is the result of multiple migrations northward through the last approximately 11,600 years that gradually colonised a landscape rendered near-sterile by the recently concluded Quaternary glaciations and shorter cold periods termed 'stadials'. The recency of Britain's flora means that the islands are populated with the tips of numerous evolutionary branches of larger lineages, each of which reliably shows much greater diversity in continental Eurasia. Resolving the phylogenetic relationships of our orchid species with any degree of reliability requires complete species-level sampling of each genus on at least a European scale — a programme that fortunately is now well underway. Even more fortuitously, it is the ostensibly most taxonomically troublesome genera that have been prioritised for study.
Ironically, the net result of being able to access an expanded number of more data-rich phylogenetic studies has not strengthened the aggregate topology shown in Fig. 1. In fact, the 2006 and 2021 trees both contain seven de facto polytomies that by definition represent areas of weakness; some of the polytomies occupy contrasting locations in the two topologies, but overall resolution has not been improved. Anacamptis and Serapias are more clearly seen as sister genera, but in contrast, the placement of both Himantoglossum and Orchis has become more ambiguous. In addition, some relationships among major groups of species remain highly contentious within Serapias (cf. Bellusci et al. 2008; Sardaro et al. 2012) and Orchis (cf. Bateman et al. 2003; Tyteca & Klein 2008; Bateman 2009; Tyteca et al. 2012). Within subfamily Epidendroideae, relationships of species within Epipactis have become better resolved (Sramkó et al. 2019), but the obligate mycoheterotrophs continue to be a widely acknowledged source of topological instability.
These persistently ambiguous relationships among some genera and species weaken our ability to interpret the evolutionary history of British and Irish orchids. However, it is important to note that they do not weaken the circumscriptions of genera originally advocated by Bateman et al. (1997, 2003). Circumscribing genera according to a combination of (1) monophyly and (2) the comparative robustness of the branch subtending the putative genus has proven satisfyingly resilient to all further well-founded analyses (e.g. Bateman 2009, 2012a). Unfortunately, most taxonomic treatments still fail to acknowledge the importance of criterion 2, and astonishingly, some even persist in ignoring criterion 1. In consequence, some authors continue to advocate separation of Coeloglossum as a monotypic genus separate from Dactylorhiza, of Nigritella from Gymnadenia, and/or of Listera from Neottia. Evidence remains unclear regarding whether 'Coeloglossum' is nested within, or is instead sister to, the rest of Dactylorhiza (cf. Devos et al. 2006; Bateman & Rudall 2018; Bateman et al. 2018a; Brandrud et al. 2020), though this uncertainty should not impact on the cast-iron case for including 'Coeloglossum' within Dactylorhiza. The same statement can be made with regard to the question of whether to include 'Nigritella' within Gymnadenia (cf. Bateman et al. 2018a; Brandrud et al. 2019). Other insupportable suggestions made in recent literature include the attempt to segregate Orchis mascula, O. spitzelii and their relatives as the new genus Androrchis, thereby separating them from anthropomorphic Orchis species such as O. simia (cf. Tyteca & Klein 2008; Bateman 2009; Tyteca et al. 2012), or to establish a new genus Odontorchis, thereby separating the rest of Neotinea from a once again monotypic N. intacta (cf. Kretzschmar et al. 2007; Tyteca & Klein 2008; Trávníček et al. 2021), or to restore a monotypic Anacamptis by separating off the paraphyletic remainder of the genus as a new genus Herorchis (Tyteca & Klein 2008). Notwithstanding possible monophyly in the majority of these cases, all of the molecular research makes clear that in each case the branch separating these 'genera' from related species is too short to warrant genus-level recognition. Also, in the case of 'Listera', its non-monophyly in the absence of Neottia s.s. is unequivocal (Bateman et al. 2005; Zhou & Jin 2018).
A further unwelcome development was the division by Delforge (2009) of Bateman et al.'s (1997) expanded Anacamptis into no less than five "genera": Anacamptis sensu stricto (A. pyramidalis), Herorchis (here much reduced from the Tyteca & Klein [2008] concept of Herorchis, becoming centred on A. morio), Paludorchis (A. laxiflora), Anteriororchis (A. coriophora) and Vermeulenia (A. papilonacea). This ultra-divisive classification of Anacamptis, which circumscribes 'micro-genera' that contain only between one and at most six bona fide species, was criticised heavily by Bateman (2009, 2012a) but nonetheless was subsequently adopted in Continental orchid monographs by not only Delforge (2016) but also Kreutz (2019), who perversely chose to reject all of the genus-level rearrangements enacted on clear molecular evidence and explicit criteria by Bateman et al. (1997, 2003). Happily, with the arguable exception of a few die-hard enthusiasts for the comfort of traditional pre-1997 generic circumscriptions (Lang 2004; Curtis & Thompson 2009), taxonomy rooted in explicit science has been dominant in Britain and Ireland throughout the last quarter-century (cf. Harrap & Harrap 2009; Stace et al. 2016; Stace 2010, 2019; Cole & Waller 2020; Stroh et al. 2022).
Lastly, when considered at the species level (reviewed by Bateman 2012b), the present Fig. 1 shows only four changes from the phylogeny presented by Bateman (2006, fig. 2). The putative island endemics Dactylorhiza ebudensis and Epipactis sancta, advocated tentatively on the basis of limited molecular evidence by Bateman (2006), are here downgraded to infraspecific taxa, whereas recent finds of single plants of Serapias vomeracea s.s. and the closely related S. cordigera add these species to the current list of potentially native orchids. Each of these four species-level cases is discussed in greater detail under its respective genus-level heading below.
'Unrooted' evolutionary tree constructed via SplitsTree4 based on 29,953 RAD-seq SNPs, showing the relationships of 95 tetraploid dactylorchids among each other and relative to their parental diploid species (boxed: 'mother' given before 'father'). Modified after fig. 2 of Bateman (2019) and a preliminary version of supplementary fig. S3 of Brandrud et al. (2020).
Dactylorhiza
Allopolyploids
The taxonomic treatment of Dactylorhiza in Bateman (2006) primarily reflected the large-scale UK-based morphometric studies of Bateman & Denholm (1983, 1985, 1989) combined with two geographically broader molecular investigations: the AFLP study of Hedrén et al. (2001) and the Sanger sequencing study of both nuclear and plastid regions that was, after repeated delays, eventually published as Pillon et al. (2007). Since 2006, Dactylorhiza has received considerably more research attention than any other native orchid genus. Much of the scientific interest has focused on the long-recognised ability of the diploids D. fuchsii (and its close relatives) and D. incarnata (and its close relatives) to repeatedly generate new species through allopolyploidy: hybridisation combined with whole-genome duplication. Comparing nuclear genomes inherited from both parents with plastid genomes inherited only from the seed-parent soon demonstrated that D. fuchsii (or, in the case of D. kerryensis, the autotetraploid D. maculata) was always the 'mother' and D. incarnata always the 'father' (Pillon et al. 2007), though even today this observation remains in need of fundamental explanation. As increasingly sophisticated sequencing technologies were applied to these plants, it became more feasible to obtain approximate dates for the origin(s) of each species, D. praetermissa having originated before D. traunsteinerioides which in turn originated before D. purpurella, whose origin may be post-glacial and most likely occurred within the British Isles (Bateman et al. 2022).
However, these insights left open two contradictory but nonetheless equally logical species concepts. Bateman (2011a; also Bateman & Denholm 2012; Brandrud et al. 2020; Bateman et al. 2022) argued that each demonstrable polyploidy event equates with a single speciation event, whereas for Hedrén (e.g. Hedrén et al. 2008; also Pedersen 1998, 2007; Kühn et al. 2019), all polyploidy events that occur between the same parental species are repeatedly generating the same descendant species, despite its unequivocal multiple origins. This taxonomic "lumper's charter" is made even lumpier by the decision of Hedrén and many other continental orchid enthusiasts to continue treating the largely diploid calcicole D. fuchsii as an infraspecific taxon within the uniformly tetraploid calcifuge Dactylorhiza maculata, so that the spotted-orchids of section Maculatae then appear to represent only a single parent of the numerous derived polyploids.
One of the more controversial aspects of Bateman (2006) was his figure 5; a deconstruction of a single distribution map purporting to be the widespread continental tetraploid 'Dactylorhiza majalis' included in the 2002 edition of the British and Irish plant atlas (Preston et al. 2002). I argued that "the map actually represents anthocyanin-rich individuals of three allotetraploid species of independent origin within Britain, none of which corresponds with the exclusively Continental D. majalis s.s." Specifically, I noted that most of the hectads mapped for the island of Ireland as D. majalis actually represented D. kerryensis (at the time referred to as 'D. occidentalis'), that those hectads mapped along the coasts of N. Ireland, Scotland and Wales were attributable to D. purpurella var. cambrensis plus 'D. ebudensis' (a taxon confined to the Hebridean island of North Uist), and that an outlying record of 'majalis' in Yorkshire represented a hybrid swarm between D. fuchsii and D. purpurella.
Two decades of further research have not only confirmed these interpretations but also launched additional molecular-based critiques of particular Dactylorhiza maps presented in Preston et al. (2002). By 2011 it had become clear that the map provided for 'D. lapponica' actually represented an anthocyanin-rich subspecies (or perhaps variety?) of the same species that had been separately mapped on the same page of the Atlas as 'D. traunsteineri' (Bateman 2011a; Hedrén et al. 2011a; Bateman et al. 2022). Bateman & Denholm (2012) renewed their previous argument that this composite taxon had a separate evolutionary origin from both D. traunsteineri in the Alps and D. lapponica in Scandinavia, and should therefore retain the name D. traunsteinerioides. In addition, they resurrected from obscurity (Wilmott 1936) the epithet francis-drucei as a subspecies to encompass the anthocyanin-rich Scottish populations that had formerly been attributed to 'D. lapponica'. The morphological relationship between subsp. traunsteinerioides and subsp. francis-drucei is clinal, intermediate populations occurring in Co. Antrim and Cumbria. Moreover, DNA analysis of multiple plants of 'D. ebudensis' by Hedrén et al. (2011a) demonstrated that the single plant analysed by Pillon et al. (2007) was most likely introgressed with intermingled D. purpurella or D. incarnata, giving the false impression that the seed-parent of 'ebudensis' was D. incarnata (wrongly given as D. purpurella by Stace 2019). Further study showed that 'ebudensis' actually has a DNA profile typical of D. traunsteinerioides and a phenotype consistent with the anthocyanin-rich end of the morphological spectrum presented by subsp. francis-drucei. Its claim to have a recent and independent evolutionary origin was also conclusively rejected. Consequently, ebudensis was downgraded from full species to a variety of subsp. francis-drucei (Bateman & Denholm 2012; Bateman et al. 2022; discussed in detail by Bateman 2019).
However, the nuclear and plastid sequencing by Hedrén et al. (2011a) had also shown that scattered populations south of a line from the Severn to the Humber that were assigned by Preston et al. (2002) to 'Dactylorhiza traunsteineri' shared the molecular profiles of adjacent populations of D. praetermissa (Bateman 2011a). Bateman & Denholm (2012) therefore assigned these species to D. praetermissa as a new subspecies that, like D. traunsteinerioides further north, specialised in occupying Schoenus fens. A subsequent morphometric comparison (Bateman & Denholm unpubl.) showed that populations of D. praetermissa subsp. schoenophila do differ morphologically as well as molecularly from those of D. traunsteinerioides, albeit only subtly. Unfortunately, schoenophila was not analysed for RAD-seq by Brandrud et al. (2020). Kreutz (2019) has recently suggested that this subspecies is not wholly endemic to England, arguing (rather unconvincingly) that it also occurs at single localities in Belgium and the Netherlands.
Despite being strongly supported by multiple data-sets, this re-circumscription of Dactylorhiza traunsteinerioides in the British Isles did not end the debates regarding its degree of distinctness from, and relationships with, D. lapponica in Scandinavia and D. traunsteineri in the Alps. A study of DNA methylation by Paun et al. (2010) clearly distinguished traunsteinerioides from traunsteineri, and in addition indicated a north–south cline within England and Scotland that mirrored the documented morphological cline. The nuclear microsatellites studied by Balao et al. (2016) placed lapponica as intermediate between traunsteineri and traunsteinerioides, the latter being the most molecularly distinct of the three species. Molecular data generated through the superior RAD-seq technique (Brandrud et al. 2020) (Fig. 2) showed traunsteinerioides to differ reliably from Continental populations but failed to distinguish the Scandinavian lapponica (sampled in Norway, Sweden, Finland and Estonia) from Alpine traunsteineri (sampled in Switzerland, Austria and Germany). Interestingly, the otherwise uniformly British traunsteinerioides clade included a single plant sampled from the west coast of Norway (arrowed in Fig. 2), hinting at the possibility that traunsteinerioides may not be wholly endemic to the British Isles. Both traunsteinerioides and traunsteineri-lapponica were at least as molecularly distinct as D. praetermissa, D. purpurella and D. majalis. It therefore came as a surprise that modelling of the RAD-seq data by Brandrud (2019) suggested that traunsteinerioides arose from within traunsteineri rather than having its own independent allopolyploid origin in the British Isles, the two taxa having separated comparatively recently.
The change of name of the Irish marsh-orchid from Dactylorhiza occidentalis to D. kerryensis was a purely nomenclatural transition, enforced by over-zealous imposition of the International Code of Nomenclature by an ICN panel who overrode a multi-author application for nomenclatural conservation of the better-known epithet. Nonetheless, the RAD-seq analysis (Fig. 2) provides further scientific evidence that the dominant Irish allotetraploid, D. kerryensis, is distinct from the remaining British and Irish allopolyploids, presumably because its founding hybridisation event involved D. maculata rather than D. fuchsii as seed-parent (Pillon et al. 2007; Hedrén et al. 2011a; Brandrud et al. 2020). It is also the only orchid species able to make an irrefutable claim to be endemic to the British Isles — more specifically, to the island of Ireland.
To summarise, during the last decade, all four bona fide tetraploid marsh-orchid species native to Britain and Ireland have been re-circumscribed taxonomically in the light of molecular and, to a lesser degree, morphometric reappraisal as certain genotypes, phenotypes and/or regional ecotypes were transferred from one named species to another. The resulting taxonomic circumscriptions have largely been followed by subsequent authors in both Britain (e.g. Harrap & Harrap 2009; Stace 2019; Cole & Waller 2020; Stroh et al. 2022) and continental Europe (e.g. Delforge 2016; Eccarius 2016; Kühn et al. 2019), though Kühn et al. chose to treat the allotetraploids as subspecies of a hyper-diverse aggregate species Dactylorhiza majalis.
Autopolyploids and diploids
The genetics of the spotted-orchids — the diploid Dactylorhiza fuchsii and autotetraploid D. maculata — were studied in impressive detail by Stahlberg & Hedrén (2010) using nrITS and plastid microsatellites, but the structures detected in their data were complex and had few implications for the British Isles, other than indicating genetic similarities of our so-called subsp. ericetorum with populations of D. maculata in Portugal, the Faroe Islands and Iceland. Similarly, the RAD-seq-based study of Brandrud et al. (2020) mostly raised outstanding taxonomic issues among taxa that are absent from the British Isles, though their results did suggest that, on molecular grounds at least, the epithet ericetorum — still widely used in Britain and Ireland to encompass all populations of D. maculata — does not even merit the status of subspecies (reviewed in detail by Bateman 2021b). Little has been learned regarding the ecotypes of D. fuchsii since the molecular study of Pillon et al. (2007) hinted at the possibility of subtle molecular differences distinguishing the north-western var. hebridensis and south-western var. cornubiensis (though not var. okellyi) from the remaining varieties sensu Bateman & Denholm (1989).
It has long been evident that the diploid marsh-orchids traditionally aggregated as Dactylorhiza incarnata maintain an inexplicably low level of genetic variation. Late 20th century studies of allozymes demonstrated that British and Irish populations of the species reliably differed from continental populations in maintaining the b allele rather than the a allele for the 6-pgd locus (an allele that, indicatively, was inherited by the near-endemic allotetraploid D. purpurella). Hedrén (2009) then showed that most British plants of D. incarnata have the A plastid haplotype, unlike continental European plants that are dominated by the B haplotype. It was therefore no surprise that Brandrud's (2019) RAD-seq-based ordination of D. incarnata s.l. had a first axis that strongly separated British plants from all continental plants. Moreover, the British plants were arrayed along the axis as a molecular cline, those from southern England being most similar to continental plants and those from the Outer Hebridean islands of Scotland being least similar (Brandrud 2019). The only exceptions were plants of D. incarnata from western Norway, which clustered with the English plants (suggesting that broad molecular and morphological comparison of orchid populations along the eastern seaboard of the Atlantic is long overdue).
Within the British Isles, the only exceptions to the apparent molecular apartheid separating British from Continental Dactylorhiza incarnata were occasional Irish populations and rare Scottish populations containing leaf-marked plants. Such populations were traditionally ascribed to the continental subspecies (or species) D. incarnata subsp. cruenta, though more recently, some authorities have ascribed the Irish leaf-marked plants to D. incarnata var. hyphaematodes rather than subsp. cruenta, an attribution justified on the rather tenuous basis of their longer, narrower leaves (Curtis & Thompson 2009). Hedrén (2009) then showed that Alpine cruenta share with most British plants of D. incarnata the A plastid haplotype, whereas cruenta populations in Scandinavia (the type region) are dominated by the typically Continental B haplotype. Finally, Hedrén et al. (2011b) used small numbers of both plastid and nuclear microsatellite loci to show that leaf-marked plants in the famous 'cruenta' population surrounding Lough Gealain, in the Burren country of western Ireland, deviated from the genotypes of both Scandinavian and Alpine cruenta. Moreover, the leaf-marked plants at Lough Gealain showed surprisingly limited gene exchange with admixed unmarked plants and maintained lower levels of genetic variation. Hedrén et al. (2011b) accepted the morphometric conclusions of Bateman & Denholm (1985) that the marked and unmarked plants were otherwise similar phenotypically, and therefore argued that the leaf-marked plants merited only the status of variety or forma.
Yet, taken together, these observations offer an alternative viewpoint on the leaf-marked incarnatas of the British Isles. In particular, the presence of the Continental allozyme profile in leaf-marked plants only, irrespective of location within the British Isles, appears consistent with the comparatively recent arrival, and successful establishment, of continental seed. The modest genetic distinctions shown by the leaf-marked plants should be viewed in the context of the absence of such differences (at least, as revealed by old-fashioned allozyme analyses and Sanger sequencing) among the other subspecies recognised within Dactylorhiza incarnata by Bateman & Denholm (1985). On present evidence, it seems to me that subspecific rank remains justified. Determining the most appropriate epithet for these distinctively leaf-marked plants is an even more challenging task, given the perceived genetic and morphological differences among populations in the Alps, Scandinavia and the British Isles. Pending more detailed studies, it seems logical to continue referring these populations to D. incarnata subsp. cruenta s.l.
Understandably, the morphological circumscriptions of the remaining subspecies of Dactylorhiza incarnata (incarnata s.s., coccinea, pulchella, ochroleuca) continue to be challenged periodically. The narrow circumscription of subsp. ochroleuca advocated by Bateman & Denholm (1985) — requiring lips that are distinctly three-lobed and obviously yellow in colour — appears to have become widely accepted, paler creamy-yellow flowered plants with lip size and shape more typical of incarnata s.s. now consistently being placed within other subspecies. It has become increasingly clear that subsp. incarnata is more distinct in the south than the north of the British Isles, where smaller, darker-flowered plants tend to grade morphologically into subsp. coccinea. Broad versus narrow circumscriptions of subsp. pulchella are also still contested. The narrower circumscription confines this subspecies to purple-flowered populations occupying acidic habitats (e.g. Harrap & Harrap 2009; Cole & Waller 2020). The broader circumscription, encompassing all purple-flowered populations lacking leaf markings (e.g. Bateman & Denholm 1985), causes at best confusion and at worst derision in continental Europe, where most populations of D. incarnata are calcicolous but nonetheless bear purple rather than pink flowers. Interestingly, the comprehensive Dactylorhiza monograph of Eccarius (2016) regards subsp. pulchella as endemic to Britain and Ireland.
Subspecies gemmana has not yet been subjected to published morphometric analysis, but our unpublished evidence shows it to be a meaningless grab-bag of unusually vigorous populations better apportioned among other subspecies. This statement can be extended to encompass a recent assertion that dune systems in South Wales support Dactylorhiza incarnata 'subsp. lobelii', a taxon recognised 20 years ago in the Low Countries but new to the British Isles (Clark & Lewis 2018). It could alternatively be described as a 'gemmana' morph stunted in stature by its dune-slack setting. Of particular note is the fact that the taxonomist who first established lobelii as a subspecies, Pedersen (2001), did so specifically by comparing morphometrically populations of subsp. coccinea occupying Welsh dune-slacks with populations of subsp. lobelii that he regarded as being confined to similar habitats in the Netherlands and Denmark. He cited the main distinguishing features as bright crimson/ruby flowers and stem anthocyanins usually present in subsp. coccinea versus dilute reddish-purple-lilac flowers and stem anthocyanins rarely present in subsp. lobelii. However, gradations between these two extremes of pigmentation actually occur in most duneland populations of D. incarnata in the British Isles, suggesting that lobelii is better left occupying its original taxonomic pigeonhole as a variety (Vermeulen 1949).
Given the many questions still posed by Dactylorhiza incarnata, it is perhaps understandable that Cole & Waller (2020) mischievously demoted all of its subspecies to varieties; this polymorphic species is certainly ripe for deeper, multi-faceted investigation. Genetic understanding of this peculiar species should at least be enhanced by the recent successful annotation of a reference genome (O. Paun, pers. comm. 2021). More broadly, despite ongoing uncertainties, it is greatly to be hoped that something close to an optimal taxonomy has now been achieved for the genus Dactylorhiza in the British Isles — one that might even be respected by the perennially volatile community of European orchid enthusiasts.
The issue of hybridity among the notoriously promiscuous orchid species of the British flora has benefited from a recent comprehensive text (Stace et al. 2016) featuring particularly detailed accounts of hybridisation in the genera Dactylorhiza and Gymnadenia. Although most of the hybrid combinations that could conceivably occur among British and Irish orchids have already been not only recorded but also formally named as so-called 'nothospecies', a few potential combinations remained to be established, relating primarily to hybrids within Epipactis, hybrids within Dactylorhiza that involve the early-divergent D. viridis as one of the parents (a species unfortunately treated as 'Coeloglossum viridis' in Stace et al. 2016), and bigeneric hybrids between species of Dactylorhiza and the three near-cryptic species of Gymnadenia. The hybrid between Gymnadenia densiflora and Dactylorhiza praetermissa was formally described by Clark & Lewis (2011), while Bateman et al. (2017a) demonstrated the value of combining nuclear and plastid sequencing for identifying hybrids — in this case, the novel hybrid between G. densiflora and D. praetermissa. Improved identification skills mean that hybrids involving D. viridis are being found with increasing frequency, most notably in the Outer Isles of Scotland. Here, hybrids with the spotted-orchids D. fuchsii and D. maculata occur in significant numbers at some sites in the Outer Hebrides, and convincing hybrids of D. viridis with D. incarnata and D. purpurella have also been captured digitally by BSBI members.
Gymnadenia
Bateman (2006) argued for species-level recognition for three Gymnadenia taxa long recognised as components of the British flora, most commonly as subspecies, but he presented no supporting evidence. The genus has since been subjected to microsatellite study within the British Isles (Campbell et al. 2007), Europe-wide study using Sanger sequencing (e.g. Bateman et al. 2018a), high-throughput sequencing through RAD-seq (Brandrud et al. 2019) and analysis of transcriptomes (Pineiro-Fernández et al. 2019), genome size estimation through flow cytometry (e.g. Trávníček et al. 2012), biochemical analysis of their scents (e.g. Gupta et al. 2014), detailed morphometric survey (Bateman 2021a; Bateman et al. 2021b) and in-depth studies of its reproductive biology within single-species and mixed populations (e.g. Stark et al. 2011; Chapurlat et al. 2015, 2018) — all subject to recent review (Bateman 2021a; Bateman et al. 2021b).
The net result of this intensive study has been to strengthen considerably circumscription of the approximately 12 bona fide species within the genus (reviewed by Bateman 2021a). Although the order in which the species originated remains contested, thereby complicating attempts to interpret evolution in the genus, all molecular analyses have yielded congruent species circumscriptions, and have agreed that long-spurred species and short-spurred species do not form monophyletic groups, even though short-spurred species are typically more geographically restricted and occur at higher altitudes. Thus, although some traditional taxonomists continue to impose the indignity of extreme taxonomic atomisation on the three or four legitimate species of Gymnadenia section Nigritella (cf. Teppner 2004; Foelsche 2014; Hedrén et al. 2018), a stable species-level taxonomy has been achieved for section Gymnadenia that reflects both morphological and especially molecular support (Bateman et al. 2018a; Brandrud et al. 2019; Bateman 2021a).
Only species of subgenus Gymnadenia are present within the British Isles and, within this subgenus, only long-spurred species occur. Three named taxa were most commonly viewed as subspecies (e.g. Rose 1988) until they were raised to species by Bateman et al. (1997) on the basis of being characterised by contrasting ITS sequences — a decision that has received a decidedly mixed reception from botanists during the ensuing quarter-century. Although it has since been proved that all species in the subgenus can readily be separated using the simple genetic "barcode" ITS, the three British native species are undeniably challenging to distinguish using morphology; there exists considerable disparity between the respective levels of genotypic and phenotypic divergence (Bateman et al. 2018b, 2021b). Particularly surprising, but supportive of their status as distinct species, was the realisation that the three species are not each other's closest relatives within the genus, despite their close morphological similarity.
Fortunately, each of the three species has a contrasting habitat preference: Gymnadenia conopsea s.s. occupies limestone grassland, G. borealis inhabits damp heathland and rough pasture, and G. densiflora specialises in alkaline marshes and flushes (reviewed by Meekers et al. 2012). In addition, at any particular latitude and altitude, G. densiflora flowers 2 – 3 weeks later than the other species. Indeed, the differentiation between Scandinavian populations of G. conopsea s.s. and G. densiflora appears to be maintained by selection on phenology rather than floral morphology (Chapurlat et al. 2020). At the genomic level, the three species can be differentiated through subtle differences in genome size; in addition, G. conopsea s.s. includes some autotetraploid populations (Trávníček et al. 2012).
The well-sampled morphometric survey by Bateman et al. (2021b) allowed the degree of morphological differentiation between the three British and Irish species to be assessed with precision, as well as the effectiveness of individual morphological characters for distinguishing among them. Their initial results showed no overlap in overall morphology between individuals of Gymnadenia borealis and G. densiflora, and limited overlap between either species and the morphologically intermediate G. conopsea s.s (Fig. 3). The best way to approach morphological identification in this group is to identify populations rather than individuals, examining several plants per population using a prioritised list of characters that have been demonstrated statistically to be taxonomically useful. The list is dominated by floral dimensions, subtle differences in flower colour, and leaf counts and dimensions (Bateman & Denholm 2019; Bateman et al. 2021b). Some of the morphological characters previously used to distinguish among these taxa (Rose 1988; Stace 2019) proved to be either ineffective or be represented by suboptimal thresholds. When identifying unknown populations it is best to consider the three possible species consecutively, beginning with G. borealis and ending with G. conopsea s.s. as a null hypothesis.
Plot of the first two principal coordinates for mean populations of all three species of Gymnadenia native to Britain and Ireland, with G. densiflora subsp. cf. friesica differentiated as "G. densiflora s.l.". Derived from a diverse matrix of 39 morphological characters. Parenthetic percentages represent the proportion of the total variance accounted for by each coordinate. Characters contributing significantly to each coordinate are listed in order of decreasing importance; arrows indicate the direction of increased value. Links in the superimposed minimum spanning tree are categorised according to contrasting Gower Similarity values. Realised in Deltagraph v.7.1. Source: fig. 9 of Bateman et al. (2021a).
Unfortunately, this relatively straightforward situation is further complicated by a small number of British and possibly also Irish populations that share the flowering time of Gymnadenia densiflora s.s. but have morphologies intermediate between G. densiflora s.s. and G. conopsea s.s. Often misleadingly described in the literature as "late-flowering G. conopsea", these populations are capable of enthusiastically occupying limestone grassland and dune-slacks. Both ITS sequencing (Bateman et al. 2018a) and RAD-seq (Brandrud et al. 2019) placed these populations as a derived group within G. densiflora s.l., and they were tentatively assigned to the continental infraspecific taxon friesica (sensu Kreutz & Lewis 2015; Lewis 2015) by Bateman et al. (2021b) as G. densiflora subsp. cf. friesica. This provisional nomenclatural assignment requires confirmation through DNA sequencing of plants from the Dutch Frisian Islands.
Sites in the British Isles supporting two of these species represent a more serious challenge to identification and require careful scrutiny. It is important to note that hybrids between these three species cannot be identified with confidence using morphology alone; molecular study would be required.
Platanthera
Perception of the genus Platanthera in the British Isles has changed little since Godfery's (1933) landmark monograph. Field botanists are well aware that, although they are broadly similar in morphology, P. bifolia is most readily distinguished from P. chlorantha by its pollinaria, which are arranged in parallel fashion rather than converging upward — the consequence of the viscidial discs being widely separated by a stigmatic surface that is over-expressed during flower development (Bateman et al. 2012). Most other characters identified morphometrically by Bateman et al. as helping to distinguish the two species also relate to the gynostemium. The two British and Irish species retained their morphometric distinctiveness when placed in the context of other European species of Platanthera (Bateman et al. 2014); the morphological discontinuity separating the two species was occupied only by putative hybrids, which resembled P. chlorantha more closely than P. bifolia.
It was initially assumed that the identity of these hybrids could be rendered certain by single-gene (Sanger) sequencing of the popular nrITS region, which is capable of distinguishing reliably almost all bona fide species of orchid native to Europe. However, the two British species of Platanthera proved to share the same two ITS ribotypes, which differed by only one single-base-pair transversion; moreover, both ribotypes often co-occurred in the same individual. In addition, analysis of eight plastid genes failed to identify any differences between the species (Bateman et al. 2012). Such results are more typical of conspecific individuals, suggesting either that P. bifolia and P. chlorantha are indeed conspecific (a conclusion unlikely to find favour with morphologically inclined field botanists) or that one species arose from the other relatively recently, the two species most likely remaining linked through ongoing gene flow. If they are genuine species, eventual application of more advanced 'next-generation' sequencing techniques should prove capable of revealing reliable distinctions within the nuclear genome.
Bateman et al. (2012) documented considerable morphological variation within both species, much of which is surely epigenetic and/or ecophenotypic — the result of differences in the vigour of individual plants, and in environmental influences such as whether they occur in sun or at least partial shade. However, the morphometric variation detected appeared to have more structure within Platanthera bifolia, relating in particular to its long-recognised willingness to occupy habitats as divergent as damp acidic heathlands on the one hand and dry calcareous beechwood hangers on the other (e.g. Summerhayes 1951). Platanthera chlorantha is more reliably a plant of at least mildly calcareous grasslands and especially open scrub or woodland margins, where it sometimes co-occurs (and hybridises) with the woodland ecotype of P. bifolia.
However, recent research in continental Europe has further complicated our understanding of the Platanthera bifolia aggregate (reviewed by Buttler 2011; Lorenz et al. 2015; Tyteca & Esposito 2018). Firstly, supposed isolation by contrasting pollinator spectra and contrasting pollinator behaviour when interacting with the flowers have both proven to have been greatly exaggerated; there is actually significant cross-pollination and cross-fertilisation between individuals of the two species (e.g. Claessens et al. 2008; Bateman et al. 2012; Esposito et al. 2017, 2018; Swainbank 2017). In a study of a mixed population in Estonia, Mötlep et al. (2021) found that gene flow was unidirectional, pollinaria only passing from P. bifolia to P. chlorantha. Secondly, given their genetic similarity, it was predictable that artificial crossing experiments would reveal full compatibility between P. bifolia and P. chlorantha (Esposito et al. 2017, 2018; Mötlep et al. 2021).
Intriguingly, research groups based in Germany and Belgium have both concluded, from separate studies combining morphometric and DNA-based analyses, that what has long been viewed as Platanthera bifolia actually represents two species. However, as summarised by Tyteca & Esposito (2018), each of the two groups appears to have recognised a different segregate from P. bifolia s.l. Platanthera bifolia s.s. is judged to be the ecotype favouring open habitats and damp acidic soils. Compared with P. bifolia s.s., the two segregates have slightly greater separation of the viscidia (i.e. broader stigmas sensu Bateman et al. 2012) and other subtle morphological differences. In contrast, the two segregates closely resemble each other in morphology. The segregates differ primarily genetically, P. fornicata (sensu Tyteca & Esposito 2018) resembling P. bifolia s.s. but P. muelleri (Baum & Baum 2017; Durka et al. 2017) seemingly being more genetically divergent from P. bifolia s.s. than P. bifolia s.s. is from P. chlorantha, and potentially having evolved from P. fornicata (Tyteca & Esposito 2018). As well as genetics, P. fornicata also resembles P. bifolia s.s. in phenology, scent composition, and associated spectra of both pollinators and mycorrhizae (Esposito et al. 2016, 2017, 2018). Reports on the ecological preferences of these segregates are ambiguous, but imply occupation of grassland and especially woodland habitats on calcareous soils. The distribution of P. muelleri has been estimated to extend from the Low Countries to Austria, and perhaps northward to Scandinavia (Durka et al. 2017), though I consider it unlikely to have been successfully distinguished from P. fornicata in the field. Earlier suggestions that P. fornicata represented adaptation to pollinator-mediated selection (Esposito et al. 2018) were rapidly undermined by the results of a subsequent, more multi-faceted analysis (Ambroise et al. 2020).
The key question now becomes what implications, if any, these studies have for our understanding of Platanthera bifolia as it occurs in the British Isles. Presumably, the "true" P. bifolia s.s. encompasses those British and Irish populations that occupy acid heathland. But the less widespread woodland ecotype, found most frequently in southeast England, may actually be attributable to P. fornicata or P. muelleri. In the morphometric ordination of British Platantheras by Bateman et al. (2012, their fig. 3), plants of P. bifolia s.l. sampled from acid heaths, calcareous downland and calcareous woodland were largely separable, the latter proving the most similar to putative hybrids with P. chlorantha (Fig. 4). It seems likely that these populations (represented in Fig. 4 by two populations from Kent and one from Oxfordshire) are consistent with the continental P. muelleri. I agree with Tyteca & Esposito (2018) that what is now most needed is a trans-European comparison of P. chlorantha, P. bifolia, P. fornicata and P. muelleri that employs both next-generation sequencing and detailed morphometrics, aiming to determine whether either or both segregates are genuine species and to clarify their respective distributions. These problems will not be solved by pursuing further parochial studies.
Plot of the first and third principal coordinates for 37 diverse morphological characters measured in 21 British and Irish populations of Platanthera bifolia and/or P. chlorantha. Parenthetic percentages represent the proportion of the total variance accounted for by each coordinate. Characters contributing significantly to each coordinate are listed in order of decreasing importance. All characters for the first coordinate increase in quantitative value towards the right of the plot. For the third coordinate, lateral sepal position is lower in the acidic populations of P. bifolia, whereas prostrate leaves are more frequent in downland populations. Realised in Deltagraph v7.1. Data derived from Bateman et al. (2012).
Epipactis
Unlike Dactylorhiza, Gymnadenia and Platanthera, Epipactis has not yet been subjected to detailed morphometric study across Europe; unfortunately, taxonomy in this challenging 'critical' genus still largely reflects traditional approaches to morphological comparison. The technical conservatism exhibited by morphologists contrasts strongly with the intensive DNA-based research that has been conducted on both these plants and their mycorrhizae (a more detailed review of the British and Irish Epipactis is provided by Bateman 2020a).
Even when reviewing this genus in the early 2000s, Bateman (2006) was able to draw on extensive allozyme data plus DNA barcode sequences generated by Squirrell et al. (2001, 2002) and Hollingsworth et al. (2006). Among British and Irish taxa, these molecular data largely confirmed traditional species boundaries refined during the mid-20th century by such luminaries as Godfery (e.g. 1933) and Young (e.g. 1962). However, the early molecular data also confidently rejected some of the later 20th century British taxonomic innovations, such as establishing the putative endemic species Epipactis 'youngiana' (Richards & Porter 1982) and downgrading E. dunensis from a widely accepted species to a mere variety of E. leptochila, thereby unifying their formerly allopatric distributions in northern England and Wales versus southern England (Stace 1991; Richards 1994, 1998).
In addition, Hollingsworth et al. (2006) showed that a small population closely resembling Epipactis dunensis in both morphology and molecules, and confined to a dune system on the Northumbrian island of Lindisfarne, deviated from E. dunensis in lacking a single molecular character, specifically a plastid indel; its absence was considered more typical of E. leptochila. This molecular distinction was sufficient to encourage Bateman (2006) to tentatively support the elevation of this species to a full species, E. sancta — a nomenclatural combination enacted four years earlier by Delforge & Gévaudan (2002) on the basis of a few (admittedly rather trivial) morphological distinctions. Kreutz (2007) then demoted sancta to a subspecies of E. dunensis, simultaneously establishing a parallel subspecies tynensis to accommodate inland populations of E. dunensis that were reputed to be subtly morphologically distinct.
Research emphasis on the genus then switched to continental Europe. Breeding experiments conducted by Brys & Jacquemyn (2016) demonstrated that, among several closely related species of Epipactis, the more strongly autogamous taxa showed as expected lower inbreeding depression, greater outbreeding depression and a greater capacity for autonomous selfing. A particularly detailed series of studies focused on populations of E. helleborine, most commonly referred to as subsp. neerlandica, occupying dune systems along the North Sea coasts of the Low Countries, which appeared to be actively transitioning toward greater autogamy. Modest genetic deviation from E. helleborine subsp. helleborine was attributed to ongoing selection pressures (Jacquemyn et al. 2018, 2020), though spectra of mycorrhizal associates also proved to differ considerably between the two subspecies (Jacquemyn et al. 2016); it is an interesting question whether this difference played a role in the evolution of subsp. neerlandica. These studies were of more than academic interest to British botanists because throughout the last quarter century there had been speculation, focused on the extensive dune system at Kenfig in South Wales, that E. helleborine subsp. neerlandica occurred in Britain (e.g. Lewis et al. 2009).
This and many other questions were answered by the well-sampled, RAD-seq based molecular study of Sramkó et al. (2019) (Fig. 5). Subspecies status for Epipactis helleborine neerlandica proved consistent with the resulting phylogenies and, rather to my surprise, samples from Kenfig did indeed prove to be unequivocally assignable to this taxon. This subspecies should now be sought in other British and Irish dune systems. In addition, the sample of 'subsp. tynensis' clustered closely with other English samples of E. dunensis, suggesting that varietal status is more appropriate than subspecies. More broadly, it became clear that the genus Epipactis has in recent years been seriously over-split by some alpha-taxonomists, and also that no Epipactis species is genuinely endemic to the British Isles. We were able to synonymise genetically similar populations from Britain into mainland European populations that had been wrongly allocated younger species-level epithets (Fig. 5). Only then is it possible to understand that, within continental Europe, E. purpurata extends as far east as Bulgaria, E. leptochila as far as Russia, E. dunensis as far as Hungary, and E. phyllanthes at least as far as Turkey; each of these species most likely originated in eastern Europe or Asia Minor (Sramkó et al. 2019; Bateman 2020a).
Maximum Likelihood phylogram depicting the evolutionary relationship of the studied Epipactis plants based on RAD-seq data analysed using RAxML. Samples of the ancestral species E. helleborine subsp. helleborine are highlighted in red. Re-circumscribed species are placed in green boxes and subspecies in blue boxes; taxa occurring in the British Isles are asterisked. Collapsed branches attracted approximate likelihood ratio test support values of less than 80% and dashed branches achieved only 80 – 90% support. The red asterisk marks the only branch to have received meaningful support from the previous study of targeted nuclear and plastid genes by Bateman et al. (2005). Source country codes: AT, Austria; BE, Belgium; BG, Bulgaria; CH, Switzerland; CY, Cyprus; DE, Germany; DK, Denmark; FR, France; GB, Britain; GR, Greece; HU, Hungary; LV, Latvia; NL, Netherlands; PT, Portugal; RO, Romania; RS, Russia; SK, Slovakia; TR, Turkey. Modified after fig. 2 of Bateman (2020a), data from fig. 3A of Sramkó et al. (2019).
Although no support was found for any of the local endemic 'microspecies' of Epipactis that have become beloved of many continental alpha-taxonomists, such highly questionable taxa continue to be described at an alarmingly accelerating rate (e.g. Ardelean et al. 2018; Hertel & Presser 2019; Kreutz et al. 2021; Lobas et al. 2021). A classic example was provided by Kreutz et al. (2020), who chose to formally describe a small population in the Chiltern Hills as a new variety of a supposed species not previously reported from the British Isles, E. neglecta. This decision was taken in the face of clear evidence from Sramkó et al. (2019) that 'neglecta' is a synonym of E. leptochila, and that this particular population had already been shown to most likely be of hybrid origin between E. leptochila and E. helleborine (Bateman 2021c). Putative examples of this hybrid combination long known in Oxfordshire were recently subjected to morphometric analysis (Swainbank & Boseley 2021).
We now have a far clearer picture of evolutionary pattern and process within the Epipactis helleborine group, which appears to be in the midst of a genuine evolutionary radiation, new taxa being derived largely from within the frequent, widespread, ecologically tolerant ancestral species. Epipactis helleborine subsp. helleborine is evidently a profoundly paraphyletic plexus from within which emerge several monophyletic lineages of contrasting degrees of genetic distinctiveness, each showing at least moderately increased levels of autogamy. Several features, both intrinsic and extrinsic, encourage autogamy, but geitonogamy (transfer of pollinaria between different flowers on the same inflorescence, thus having the same genetic consequences as autogamy within a single flower) means that the majority of successful pollination events in all taxa constitute selfing, showing that the emphasis placed by most observers on a supposed crucial distinction between "allogams" and "autogams" is misconceived (Bateman 2020a). The less allogamous taxa appear less ecologically tolerant than E. helleborine subsp. helleborine, though some of its descendant lineages are capable of occupying habitats outside its tolerances, particularly with regard to ambient light levels and soil type. Such habitat shifts could be encouraged by contrasting mycorrhizal associations (Schiebold et al. 2017), which may eventually prove to be better adapted to the novel terrains.
Orchis s.s.
Among the four species of anthropomorphic Orchis species occurring in England, O. simia and O. militaris have each become confined to three or fewer extant native localities (Stroh et al. 2022). Other localities are still occasionally discovered but they are few and short-lived. A single plant found in a gravel pit in Hertfordshire in 2016 formed a modest clump (Fig. 6G) before being top-dressed with several tonnes of gravel in 2020. In 2021, a single plant of O. simia found in West Sussex (Clark 2021; Stroh 2021) bore flowers that shared with those of plants at the native Oxfordshire site the unusual characteristic of bearing only a few small, localised clusters of pink-stained papillae on the monkey's 'chest' (Fig. 6F). Orchis purpurea and O. anthropophora have become largely confined to the North Downs of Kent (and Surrey in the case of O. anthropophora), though each still maintains a few small outlying populations elsewhere in southern England. Both species are declining, O. purpurea slowly but O. anthropophora rapidly (Jacquemyn et al. 2011a; Walker et al. 2017). All four species prefer chalk soils.
Recently discovered populations of orchid species either new to, or rare in, the British Isles. A Serapias parviflora, Cornwall, 2008; B Serapias cordigera, South Yorkshire, 2021; C Serapias vomeracea, East Kent, 2020; D Serapias lingua, Essex, 2020; E Spiranthes romanzoffiana, Cardiganshire, 2020; F Orchis simia, West Sussex, 2021; G Orchis militaris, Hertfordshire, 2019. photos: a, c, d ian denholm; b sean cole; e jess stone; f matt eade; g richard bateman.
The comparative rarity of anthropomorphic Orchis species in Britain does not prevent them from producing occasional hybrids. These usually occur in very small numbers (reviewed by Bateman et al. 2008; Stace et al. 2016; Cole & Waller 2020), in contrast with some of the extensive hybrid swarms that routinely occur in areas such as southern France where these species are far more frequent (Kretzschmar et al. 2007; Jacquemyn et al. 2012; Bersweden et al. 2021). However, the appearance in 1999 of two flowering plants of O. purpurea in the midst of Britain's largest surviving population of O. simia led to the first flowering of their F1 progeny in 2006; by 2021, the number of F1 plants and putative backcrosses in flower exceeded 130. An integrated study of the genetics and morphometrics of the population (Bateman et al. 2008) supported previous research suggesting that hybrid terrestrial orchids inherit more of their phenotype from their mothers than their fathers, though the converse bias was shown in the genotype. The study also showed that the O. purpurea plants had probably originated in continental Europe rather than the British Isles, and that both ribotypes and plastid haplotypes found in the supposedly genetically pure population of O. simia actually more closely resembled those typical of O. militaris, which until the 20th century grew alongside O. simia in the Goring Gap of the River Thames. Evidently, there was extensive introgression among anthropomorphic Orchis species in historical times, O. militaris apparently acting more often as the donor than as the recipient (Bateman et al. 2008). Certainly, these results demonstrated that both intrinsic and extrinsic reproductive barriers are weak within this group.
Hybrids with Orchis anthropophora continue to appear periodically at the long-established native Kent site for O. simia, but a less welcome occurrence was the flowering in 2014 of three such hybrids within a small Hampshire population of O. anthropophora. The fact that they emerged in the absence of O. simia strongly suggests a mischievous case of artificial pollination (Cole & Waller 2020), thereby throwing unwarranted suspicion on other occurrences of such hybrids that are more likely to have a legitimate natural origin.
More extensively sampled studies of hybrid zones among anthropomorphic Orchis species were conducted in Belgium (Jacquemyn et al. 2012) and southern France (Bersweden 2021; Bersweden et al. 2021). Both of these studies elected to employ petal dimensions as a short-cut proxy for overall phenotype but nonetheless found a reasonable positive correlation between their chosen styles of morphometric and population genetic analyses. Introgression proved to be most common between the sister-species O. purpurea and O. militaris; backcrossing of primary hybrids occurred preferentially with O. militaris in France (Bersweden et al. 2021) but preferentially with O. purpurea in Belgium (Jacquemyn et al. 2012). Such genetic complexities are now precluded within the British Isles by the decline to mutual allopatry of O. purpurea and O. militaris, though the genetic traces of past hybridisation events involving 'ghost' lineages intriguingly persist (Bateman et al. 2008).
Serapias
Arguably the two most taxonomically controversial genera in the European orchid flora, certainly within the Mediterranean realm, are Ophrys and Serapias. In Britain, we are fortunate to possess as confirmed long-term natives only four species of Ophrys, each representing one of the nine lineages that are unequivocally distinct both morphologically and molecularly (Bateman et al. 2018b). Their straightforward taxonomy in Britain stands in stark contrast to the nightmare of trivial complexity evident among taxa representing the genus in the Mediterranean.
In contrast, the history of the genus Serapias in Britain cannot be traced any further back in time than an unvouchered record of a single plant of S. cordigera neglecta reported from a field in the Isle of Wight in 1918. Another 71 years passed before a small population of the autogam S. parviflora was found in a scrubby pasture above a Cornish cliff (Madge 1994; Rich 2003) (Fig. 6A). Sadly, this population was lost, apparently through a period of intense overgrazing that occurred in 2009. In 2021, not one but two further populations of this species were discovered; two plants tentatively attributed to this species that were found in rough grassland marginal to an extensive garden in Suffolk (S. Cole, pers. comm. 2021) and, more bizarrely, nine flowering plants found on the roof of an eleven-floor office block in central London. The plants occupied a 'green roof' composed of Sedum mats that were installed in 2009 (BSBI DDb 2021).
The history of Serapias lingua in Britain is broadly similar to that of S. parviflora. A single plant flowered on the margin of a Guernsey golf course for a single season in 1992. The first mainland occurrence consisted of three plants discovered in a coastal hay meadow in South Devon in 1998 (Lang & Spalton 1998); this population survived until at least 2003, peaking at nine plants (e.g. Harrap & Harrap 2009). The third and largest population of at least 120 plants, a number presumably reflecting the ability of this species to undergo rapid vegetative spread via short stolons, was discovered in rough grassland in North Essex in 2017 (Waller & Cole 2017; Adams 2018; Greenwood 2022) (Fig. 6D); it still survives in 2021 but the site is under serious threat of housing development. The uniform morphology of the plants suggests clonality. Although this species was wrongly accused of being self-incompatible by Adams (2018), it seems likely that the colony was established through a single seed or tuber and hence is genetically impoverished. Suggestions that the population may, in any case, fail to reproduce due to the absence from Britain of its pollinating carpenter bee (Adams 2018) rest on the questionable assumption of reliance on a single pollinator, though admittedly, levels of fruit set observed in the Essex population are not encouraging.
No less than three 'microspecies' within the Serapias vomeracea–cordigera complex have made guest appearances in Southern England since the early 20th century. The first member of this group to be reported was an undocumented occurrence in 1918 of a plant attributed to S. neglecta, found improbably in a cornfield on the Isle of Wight. Three plants of S. cordigera flowered in a disused chalk quarry in East Kent in 1996 and 1997, and in 2021 a single flowering plant of S. cordigera appeared in a small and otherwise undistinguished suburban lawn near Sheffield, South Yorkshire (Fig. 6B). Lastly, a single robust plant of S. vomeracea appeared in chalk soil among open scrub on a grassy roadside verge in East Kent in 2020 (Kitchener et al. 2021), increasing to two flowering plants in 2021 (Fig. 6C).
All arrivals of orchid species new to the British Isles will inevitably generate scepticism regarding their mode of origin, given that several possible explanations range from wholly natural aerial transport of dust-seeds from continental populations through to deliberate planting of tubers or rhizomes. There is circumstantial evidence that successful long-distance dispersal of orchids via seeds is the exception rather than the rule (e.g. Devey et al. 2009). In the case of Serapias, perhaps the strongest evidence of a natural northward migration, presumably in response to climate change, is the number of genuine species of the genus that appear to have arrived in England during the last quarter-century. The case for the defence is strengthened by the ability of Serapias species, as demonstrated in their Mediterranean heartland, to rapidly colonise a wide range of habitats. Serapias is particularly adept at invading semi-natural and even wholly anthropogenic terrains, which means that the diverse range of rather mundane habitats colonised by the genus in England should not wholly surprise us. Another question that we might reasonably ask is the proximity to England of the closest natural population of the relevant species. Most of the Serapias species hypothesised to have migrated to England have distributions that stretch northward from the Mediterranean Basin along the west coast of France; the exception is S. neglecta, which comes no closer to Britain than the Cote d'Azur. Serapias vomeracea reaches as far north as Bordeaux, whereas S. cordigera, S. parviflora and S. lingua all extend northward as far as Brittany (Bournérias & Prat 2005), a mere 160 km from the English West Country as the crow flies. The issue of inferring the origin and mode of transport of such unexpected arrivals is discussed further below, under "The complicating factor of human intervention".
Reproductive biology
Most British and Irish orchids reliably interact with pollinating insects above ground and mycorrhizal fungi below ground; these autecological relationships are increasingly seen as crucial to both understanding the evolution of orchid species and developing well-informed conservation recommendations.
During the present review period, pollination biologists have benefited greatly from the superb synthesis of modern taxonomy, floral morphology and pollination observations that is The Flower of the European Orchid (Claessens & Kleynen 2011). This and other studies have begun to employ valuable technical developments such as automated image capture and field collection of headspace volatiles emitted by orchid flowers. Nonetheless, it remains a serious challenge to gather robust data, throughout the necessary 24-hour cycle and entire flowering period, taken from many populations across the entire geographical distribution of the target species. Without such data, statements regarding pollinator spectra remain near-typological assumptions rather than hard facts.
It has become increasingly popular to categorise European orchids according to four reproductive modes: nectar reward, sexual deceit, food deceit and autogamy (e.g. Vereecken et al. 2010). Although providing a useful framework, these categories are inevitably over-simplifications. Strategies adopted by nectar-rewarding orchids range from large reservoirs held in labellar spurs, through significant quantities exuded onto the labellum surface, to small pin-point sources of presumed nectar. Much interesting work has elucidated the often complex chemical compositions of the volatile bouquets emitted by rewarding orchids (e.g. Chapurlat et al. 2020) and the pseudo-pheromones emitted by sexually deceitful orchids (e.g. Ayasse et al. 2010). More recently it has been suggested that food-deceitful orchids may also exude species-specific volatiles (Wróblewska et al. 2019). Use of autogamy as a distinct reproductive category has been heavily criticised in recent years because morphologically-based inferences can now be compared with genetic patterns that directly reflect degrees of gene flow. In particular, self-fertilisation of a single flower has the same genetic consequences as the transfer of pollinaria between different (but genetically identical) flowers of the same inflorescence. Termed geitonogamy, this is a frequent occurrence in many orchids (e.g. Kropf & Renner 2008; Bateman 2020a); most orchids lack strong inherent sterility barriers and so cannot function as obligate allogams with assured outbreeding. Interesting patterns emerge when closely related species pairs diverging in pollination mode are compared for frequency of fruit set (Fig. 7). It becomes clear that so-called autogams have on average the same percentage fruit set as supposedly dominantly outbreeding rewarding species (75 – 80%). In contrast, and as expected, non-rewarding deceitful species rarely exceed 30% fruit set. Lussu et al. (2019) applied the primarily zoological "lock and key" hypothesis to European orchids, arguing that the fit between the sizes of pollinaria and stigmatic surface is on average closer in food-deceptive species than in sexually-deceptive species.
Meta-analysis of average percentage fruit set (± sample standard deviation) for a total of 193 populations spanning four pairs of western European orchid species, each pair collectively showing two of the four contrasting pollination modes available. All taxa other than Anacamptis coriophora occur in the British Isles. Data summarised from appendix 2 of Claessens & Kleynen (2011).
However, pollination research is now coming of age, and several recent studies have highlighted the dangers of over-generalisation. Trunschke et al. (2020) found that moths differing substantially in tongue length interacted with Platanthera bifolia populations differing substantially in spur length to generate complex and contrasting patterns of pollinarium removal and deposition. Ambroise et al. (2020) studied gynostemium morphology in a mixed population of two morphs of P. bifolia plus the larger-flowered P. chlorantha, and concluded that selection could not explain the persistence of the morphologically intermediate phenotype of P. bifolia. Studying the scent of Gymnadenia conopsea s.s. in Scandinavia, Chapurlat et al. (2018) observed substantial differences in both composition and emission rate between daytime and night-time, the latter showing greater contrasts among populations. Their subsequent study comparing selection on flowering time and floral morphology in G. conopsea and the later-flowering G. densiflora (Chapurlat et al. 2020) revealed no significant stabilising selection and no divergent selection on floral features; this was confined to flowering time in a minority of the study populations. Similarly, Scopece et al.'s (2007: 1) three-year study of several phenotypic features in Orchis mascula and O. pauciflora "found weak directional selection and marginally significant selection gradients … with significant intra-specific differences in selection differentials across years", implying that "pollinator-mediated selection in food-deceptive orchids can change in strength and direction over [short] time[-scales]." Cumulatively, these studies suggest that natural selection resembles a spotlight, constantly roving around the ecological stage occupied by the species in question but rarely alighting on a particular functional feature for a length of time sufficient to influence the species' evolution.
Pollinator specificity also remains a much-debated topic, particularly in groups where it is considered comparatively strong. Perspectives on the archetypal sexually deceptive genus, Ophrys, range from perceiving a genuine ongoing adaptive radiation into several hundred species that have only one to few pollinator species (e.g. Baguette et al. 2020) through to circumscribing just nine genetically robust species that tinker trivially, transiently and largely randomly with their unusually complex phenotype, local microevolution rarely leading to speciation (Bateman 2018; Bateman et al. 2018b). The conservation implications of such radical interpretational contrasts are profound. For example, Hutchings et al. (2018) demonstrated progressive divergence in England between the observed peak flowering time of Ophrys sphegodes and the average time of emergence of the naïve solitary bees (Andrena nigroaena) that are widely considered its predominant pseudo-copulatory pollinator. They argued that the divergence most likely reflected anthropogenic climate change and could result in persistent reproductive failure of this orchid in Britain. However, such a catastrophic outcome appears far less likely if, as was documented by Breitkopf et al. (2013) in Italy, O. sphegodes actually regularly attracts as pollinators other species of Andrena. Happily, southern England supports a further 67 species of Andrena, suggesting that switching between preferred pollinators may well prove feasible for this species, whose English populations presently remain resilient (Stroh et al. 2022).
Artificial reciprocal crossing is an under-used approach to understanding orchids, and tends to be employed primarily as a means of determining seed fertility in the resulting F1 generations across a spectrum of congeneric species (e.g. Scopece et al. 2007; Brys & Jacquemyn 2016). However, it has much greater potential if analysts have the patience to both breed additional generations (F2, F3 generations and backcrosses) and record in detail the resulting phenotypes so that heritability patterns can be documented (Haggar & Malmgren 2012). Even pairs of species lacking overlap in flowering periods can be crossed successfully, through the simple expedient of freezing the pollinaria of the earlier flowering species (e.g. Antonetti et al. 2021).
Studies that attempt to relate specific changes in phenotype to specific changes in genotype also remain under-represented but show great promise. Sedeek et al. (2016) were able to relate a change in the composition of the pseudo-pheromone cocktail between Ophrys sphegodes 'microspecies' to an amino-acid substitution in a biochemical cascade involving alkene synthesis, and Kellenberger et al. (2019) identified a similarly small genetic modification that impacted on anthocyanin synthesis and thereby underpinned a radical change in flower colour in the alpine Gymnadenia rhellicani. Balao et al. (2017) were able to identify via transcriptomes those genes that were under strongest selection in Dactylorhiza fuchsii and D. incarnata, reflecting both biotic and abiotic stresses and emphasising the significance of their micro-environmental context. Their study provides a useful reminder that day-to-day maintenance of a plant is at least as important to its success as reproductive competence, which effectively constitutes the icing on the evolutionary cake.
Mycorrhizal associations
During the 21st century, a combination of improved sampling strategies and DNA-based taxonomy (focused on ITS sequences) has greatly improved our knowledge of the mycorrhizal fungi that inhabit the roots and seeds of European orchids, with important implications for conservation. Large numbers of fungal species can occur in intimate contact with a single orchid, the species composition of this spectrum undergoing striking changes during a single growing season as the roots grow, mature and senesce (Kohout et al. 2013). Often, the fungi that stimulate seed germination are not those that assist the mature roots, and differences have been noted in the fungal associates of juvenile plants relative to mature flowering plants, which tend to be less dependent on their mycorrhizal partners for carbon input (Jacquemyn et al. 2012; Roy et al. 2013). Associated fungal communities can differ markedly within the same species when growing in contrasting habitats (Jacquemyn et al. 2016), and some studies suggest that, among closely related orchid species, those that are most frequent and widespread form mycorrhizal associations with a wider spectrum of fungi (Bateman et al. 2014). However, other studies have suggested that the diversity of fungal partners is less important than finding partners that are both frequent and widespread (e.g. Jacquemyn et al. 2011b).
Most orchidoid orchid species are tuberous autotrophs, whereas most neottioid orchids are rhizomatous mixotrophs or obligate mycoheterotrophs. Grassland orchids typically rely least on their mycorrhizal partners, which are usually basidiomycetes of the Tulasnellaceae or Ceratobasidiaceae (Jacquemyn et al. 2011b, 2012; Bailarote et al. 2012; Kohout et al. 2013). Mixotrophic species — those gaining C through both photosynthesis and mycorrhizal associations — typically obtain only a minority of their C and N from fungi (e.g. Roy et al. 2013). Obligate mycoheterotrophs such as Neottia nidus-avis use their fungal plumbing to tap into solar energy generated by the surrounding trees, obtaining most of their C and N through this essentially parasitic route (Bidartondo et al. 2004; Roy et al. 2013). They are far more likely to show strong preference for a few closely similar (usually ascomycete) fungal partners (Bailarote et al. 2012); it is possible that the orchid–fungus relationship becomes sufficiently entrenched to offer opportunities for genuine co-evolution. There is evidence of limited movement of C within mixotrophic orchid plants; in Cephalanthera damasonium, C derived from mycorrhizae remains in the below-ground organs while the above-ground parts generate the bulk of their C through photosynthesis (Lallemand et al. 2019).
A comparison of taxa within the genus Epipactis by Schiebold et al. (2017) was particularly effective at demonstrating correlations between the habitat preference, the taxonomy of the associated mycorrhizae and the proportion of the plant's C and N that are derived from those fungi (Fig. 8). Marsh-dwelling species of Epipactis sect. Arthrochilum resemble in isotope composition pure autotrophs and associate mainly with endomycorrhizal rhizoctonias. Species most typical of dry soils and open or more often partially shaded habitats, such as E. helleborine s.s., obtain significant amounts of both C and N from their fungal partners, which are dominantly ectomycorrhizal basidiomycetes but also include some ectomycorrhizal ascomycetes. Species that have evolved from E. helleborine and are best adapted to deepest shade, such as E. leptochila, obtain a greater proportion of their total C and N from their fungal partners, which tend to be ascomycetes rather than basidiomycetes.
Mean enrichment factors for 13C and 15N for western European Epipactis species compared with the category of their main associated group of mycorrhizal fungi (inset); taxa unequivocally occurring in the British Isles are shown in blue rather than black font. Typical standard deviations about the mean for each species are ±1 – 2 horizontal units and ±2 – 4 vertical units. The green box represents mean enrichment factors ±1 standard deviation for 296 autotrophic reference plants, whereas the red box represents mean enrichment factors ±1 standard deviation of numerous other partially mycoheterotrophic orchid species associated with ectomycorrhizal fungi. The taxonomy used is that of the author (Bateman 2020a). Partly realised in Deltagraph v7.1. Data largely from fig. 2 of Schiebold et al. (2017).
Some key questions remain to be answered. It seems likely that the apparent evolutionary route to obligate mycoheterotrophy always passes through mixotrophy, but is the switch to a much narrower spectrum of tree-parasitising mycorrhizae a cause or a consequence of the invasion of new habitats that are less conducive to photosynthesis? And even more importantly, do mycorrhizae constitute the crucial element that restricts long-distance migration of orchid species? It is tempting to view the needs for contrasting fungal cohorts to first germinate the seeds, then feed the seedlings and finally maintain the mature plants as a crucial set of ecological filters constraining both local population expansion and longer distance migration (Bateman 2014). Evans & Jacquemyn (2020) similarly concluded that soil factors played a greater role than reproductive biology in dictating niche size among Epipactis species.
The converse symbiotic relationship with fungi — parasitism of the orchid by fungi operating as pathogens — has received worryingly little serious research. There have been recent outbreaks in the UK of mass deaths of orchid populations through the actions of Cladosporium and other as-yet unnamed fungi that collectively have been termed the 'black death'. At present, they are most likely to afflict cultivated populations, but there are signs that they are increasingly affecting wild populations, particularly of Dactylorhiza. The devastation currently being wrought on native trees by diseases such as ash die-back should alert us to the increasing threat that pathogens could pose to British and Irish orchids, particularly if inadvertently spread by orchid enthusiasts.
Systematic synthesis
So, having reviewed in detail both taxonomic and evolutionary progress in the 21st century, how many orchid species are believed to remain native to Britain and Ireland? To paraphrase Bateman (2006: 103), if Spiranthes aestivalis and Epipogium aphyllum are judged to be extirpated from the British Isles, Anacamptis laxiflora on the Channel Islands is viewed in a biogeographic context as being French rather than British, and if Serapias lingua, S. vomeracea and S. cordigera are allowed formal recognition as newly arrived British natives alongside S. parviflora (a species already granted official recognition as native for reasons that are not entirely clear), then the British and Irish orchid flora currently consists of 53 species spanning 20 genera (Fig. 1). Two putative isolated island "prospecies" — Epipactis "sancta" and Dactylorhiza "ebudensis" — have been downgraded taxonomically relative to the list recommended by Bateman (2006), whereas three Serapias species have provisionally been added, albeit in the absence of any serious research applied to these intriguing new occurrences. In addition, some might argue that a relatively recently discovered Oxfordshire population of Himantoglossum robertianum also merits consideration.
PART 2: CONSERVATION
Perceived changes in geographic distributions
Once taxa have been robustly and explicitly circumscribed, it becomes feasible to generate accurate distribution maps for them, albeit with a substantial delay needed to conduct rigorous field surveys employing the latest diagnostic characters. The Botanical Society of Britain and Ireland and the NERC Centre for Ecology and Hydrology (formerly the Institute of Terrestrial Ecology) have a strong track record of extracting patterns from the hectad-scale data that underpin successive Atlases (e.g. Groom 2013; Hill & Preston 2015), as well as from more focused surveys at finer scales specifically designed to detect vegetational change (e.g. Braithwaite et al. 2006; Walker 2008; Walker et al. 2017). Most notably, Kull & Hutchings (2006) were able to use Atlas 2000 to identify features of orchid species likely to encourage their decline: these included rapid life histories linked to preferences for dry soils and open habitats.
No doubt Atlas 2020 will stimulate further meta-analyses incorporating increasingly sophisticated probabilistic modelling techniques (cf. Duffy et al. 2008; Charitonidou et al. 2021; concepts reviewed by Hackel & Sanmartin 2021). A recent case-study of Ophrys insectifera (Charitonidou et al. 2022) prophesied wholesale losses in the Mediterranean Basin, to the benefit of the species in Britain. More specifically, the fly orchid is predicted to be lost from its wetland habitats in North Wales and Ireland, but to increase its abundance in southern England, particularly in the Midlands and East Anglia. However, the low coverage of its preferred habitat, beechwoods, in these heavily agricultural areas must cast doubt on the ability of O. insectifera to capitalise on this theoretical future opportunity. These concerns emphasise the likely benefits of focusing more explicitly on the British and Irish flora. In the case of the orchid family, BSBI Atlas data will be providing the basis for statistical modelling by the Greek-funded ExOrChiST (Extinction Debt of Orchids using Changes in Space and Time) project, based at the University of Ioannina, which aims to predict future distributional modifications likely to occur among British and Irish orchids in response to global change.
Pending more rigorous statistical analyses, even visual comparison of distribution maps in the two most recent Atlases (Preston et al. 2002; Stroh et al. 2022) suggests that at least one-third of British and Irish native orchid species have, during the 60-year period spanned by the three Atlases, undergone modifications to the outer envelope and centre of gravity of their respective distributions. Four such examples are shown in Figs 9 – 12, which compare distributions as mapped by the author through the BSBI Distribution Database at the close of the three time-slices represented by the three published editions of the British and Irish Plant Atlases.
Two of the four selected species are recorded in substantially increased numbers of hectads through time. Dactylorhiza praetermissa was judged to have increased in frequency by 27 ± 23% for the weighted Change Factor (CF) during the BSBI's Local Change project, which compared distributions in a 2003 – 2004 resurvey with those recorded in an original survey in 1987 – 1988 (Braithwaite et al. 2006). The three time-slices summarised in Fig. 9 reveal gradual consolidation, particularly in central England, where many of the habitats invaded during this period were anthropogenically disturbed. Also evident is occupation of northwest Wales and gradual northward migration of its northern margin along both the west and east coasts of northern England toward, but not yet quite reaching, the Southern Uplands of Scotland. Even the remarkable speed of northward migration achieved by D. praetermissa has been transcended by Ophrys apifera (CF +48 ± 21%), which shows a general pattern of progressive gap-filling across the British Isles. During just the last 20 years, O. apifera has not only migrated northward along the west and east coasts of northern England but has also leap-frogged the Southern Uplands to invade the Scottish Midland Valley (Fig. 10); conventional wisdom suggests that its speed of migration will have been assisted by its well-known enthusiasm for routine autogamy facilitating colonisation. In truth, few British orchids have strong intrinsic sterility barriers, allowing geitonogamy to function genetically as de facto autogamy. A similar pattern of expansion is shown by Anacamptis pyramidalis. Together with Epipactis helleborine, these three species are also proving adept at invading urban centres. In addition, Himantoglossum hircinum is spreading sporadically across southern England from its headquarters along the south coast, mirroring a previous distributional expansion during the 1930s that was carefully documented by Good (1936). Within the British Isles, it has recently been suggested that Dactylorhiza purpurella may have migrated to the island of Ireland only relatively recently, possibly via a two-pronged flanking approach from both southwest Scotland and southwest Wales (Bateman et al. 2022).
Turning to examples of species showing radical reductions in hectad occupation, Platanthera bifolia (CF -49 ± 40%) — a "widespread decliner" sensu Walker (2008) — shows a pattern of losses to year 2000 that appear fairly evenly distributed across the British Isles and are probably largely attributable to changes in land management (Fig. 11). In contrast, the post-2000 losses are concentrated in England, Wales and southern Ireland, thereby pushing the centre of gravity of its distribution towards northwest Ireland and northwest Scotland — a trend more in accord with climate change. This trend contrasts with that shown by the even more steeply declining Neotinea ustulata, which yielded a population re-find rate of only 31% in the survey conducted by Walker et al. (2017). Maps for this species in Fig. 12 reveal a pattern of gradual retreat to the species' three main refugia in England (Salisbury Plain, the South Downs of East Sussex, and Swaledale), suggesting that the causes underlying its decline are more complex but also that this species is inherently a poor coloniser.
Unsurprisingly, given the pressures on land-use across much of the British Isles, declines in hectad occupancy considerably outnumber expansions in Atlas 2020. Indeed, a recent analysis of Scottish records by Trudgill (2022) that sought to compensate for greatly increased mapping activity suggested that, with the possible exception of Scotland's most abundant orchid, Dactylorhiza maculata, all Scottish orchids are actually in decline. Anacamptis morio populations seem more likely to persist if located close to the English coastline, whereas Pseudorchis albida and Goodyera repens appear to be lost preferentially from their lower altitude sites (leading to substantial northward movement of the distributional 'centre of mass' of the latter: Groom 2013); both trends may reflect an increasingly continental climate. The largest category of seemingly declining species consists of those orchids that prefer well-drained alkaline soils but are vulnerable to desiccation caused by longer periods of hot, rain-free weather during spring and summer. Species apparently affected by this trend include some that are typical of woodland (Epipogium aphyllum, Epipactis leptochila, Cephalanthera species) but a majority that prefer open grassland (Dactylorhiza viridis, Herminium monorchis, Neotinea ustulata, Orchis anthropophora, Spiranthes spiralis).
The complicating factor of human intervention
Thus far, I have discussed distributional shifts as though they were a wholly natural phenomenon. However, apparent range expansions are also increasingly caused by direct human intervention.
The most obvious category is that of officially recognised reintroduction programmes, such as the much-vaunted Cypripedium restoration project (e.g. Fay & Taylor 2015). After overcoming several early setbacks, this resource-intensive, long-term project successfully established C. calceolus at several former localities across northern England, thereby relieving pressure on the one remaining natural occurrence imprisoned in its iron cage in the Yorkshire Dales. Another well-publicised deliberate reintroduction involved the spreading in localities across Kent of seed generated by artificial pollination of the one native population of Orchis simia occurring in West Kent, resulting in a now well-established population at the East Kent reserve of Park Gate Down (e.g. Harrap & Harrap 2009).
Serapias lingua was introduced into grassland at the botanic garden of Wakehurst Place, West Sussex by RBG Kew in the 1990s, and plants of S. vomeracea occupying a roadside verge in Somerset are known to have been deliberately introduced as tubers by a local landowner in 2011 (Cole & Waller 2020). The problem of inferring naturalness is particularly acute for the recent occurrence of S. parviflora on a high-rise central London green roof. Although its relatively high altitude strongly encourages belief in an origin in wind-blown seed, this species may alternatively have been imported (either as seed or tubers) with other plant species that were deliberately placed on the roof as turfs.
However, the more widely applicable categories of non-natural establishment of populations of novel orchid species rest not on planting of tubers but rather on either the deliberate introduction of seed into non-garden environments or the accidental spread of seed from cultivated plants (Bateman 2010b). Numerous precedents exist for both scenarios; garden cultivation of native orchids is increasing rapidly in popularity, often providing local sources of seed far outside the natural ranges of the cultivated species. Most cases of deliberate spreading of orchid seed into biodiverse semi-natural habitats are conducted surreptitiously, though a few practitioners — not least the late, great Francis Rose — have done so openly and thereby courted equally open criticism (e.g. Lousley 1976). Accidental spread of seed is also increasingly likely, as Ophrys and Serapias species in particular are proving themselves readily bred and cultivated by specialist gardeners. The occurrence of S. cordigera in Kent was rejected as native largely because the species was known to be cultivated a mere 300 m from its chalk-quarry habitat (e.g. Cole & Waller 2020), and this must surely be the method by which the European native S. lingua recently became naturalised in the hills above Adelaide, Australia (Conran et al. 2011).
Of course, these issues are not confined to the genus Serapias. Two recent appearances in southern England of the classic Mediterranean orchid Himantoglossum (formerly Barlia) robertianum (the Giant Orchid) have caused much speculation regarding their likely origins. To be more accurate, the population occupying a field near Braintree in Essex is known to represent a deliberate introduction, though as tubers were introduced from both France and Greece, it is not known which of these lineages was successful (S. Cole, pers. comm. 2022). Greater uncertainty surrounds the now well-established population in South Oxfordshire, first encountered approximately 12 years ago as a single plant (Temple 2011; W. Temple, pers. comm. 2022) but expanded to ten flowering plants at the time of writing. Was the orginal plant a deliberately introduced tuber? Alternatively, was seed sampled from a cultivated plant and deliberately spread at the site? Or did seed 'escape' from a local garden-grown plant? Or maybe, just maybe, the seed reached the site through high-level air currents from a natural population on the Continent? The archetypal French orchid flora (Bournérias & Prat 2005) shows H. robertianum reaching the northern limit of its distribution around Lyon, but the (notoriously unreliable) Global Biodiversity Information Facility map features approximately 100 records located in Continental Europe at latitudes higher than Lyon. For example, Kreutz (2019) speculated on the origin of a population found adjacent to a nature trail in Belgium. Certainly, this is a species known to readily colonise unappealing habitats.
In 2022, a character-rich morphometric comparison of Oxfordshire plants with populations from southern Spain, southeast France and northern Sardinia revealed a clear similarity with the French population (Fig. 13), as well as showing a limited amount of within-population variation that would be consistent with being founded by a single genotype. Genetic analyses could eventually give a clearer idea of the ultimate source of the Oxfordshire plants, particularly as unlike other Himantoglossum species, H. robertianum populations differ regionally in 'barcode' regions of the genomes such as nuclear ITS and plastid trnL-F (Sramkó et al. 2014; Bateman et al. 2017b). However, a rigorous study would require availability of a much larger number of samples of the species taken from sites spanning Europe.
Plot of the first two principal coordinates for 44 diverse morphological characters comparing a recently (re)discovered British population of Himantoglosssum robertianum with three geographically disparate Mediterranean populations previousy published by Bateman et al. (2017b). Parenthetic percentages represent the proportion of the total variance accounted for by each coordinate; lower-order coordinates offered no discrimination.
One instructive case of the deliberate broadcasting of orchid seed that occurred in a small meadow in Perthshire has thus far resulted in successful establishment of a remarkable total of 15 native orchid species (Trudgill 2017, 2019). The introduced species include Epipactis palustris, which has only four natural sites in southern Scotland, and Ophrys apifera, which only crossed the southern border of Scotland naturally in 2003; remarkably, a further five geographically disparate populations were found in southern Scotland during the period 2009 – 2012. Also broadcast in the meadow was Dactylorhiza praetermissa — a species whose range margin currently remains just south of the Scottish border but is migrating northward rapidly even in the absence of direct human intervention. Consequently, any new records of these species made in the vicinity of Perthshire must henceforth be considered suspect, and our ability to monitor natural migrations is (avoidably) weakened. There are many other such cases. A few, such as the Perthshire example, have been laudably well-documented, but most have not. Whatever their pros and cons with regard to conservation, deliberate introductions certainly complicate scientific enterprises such as the desire to monitor the effects of climate change (e.g. Bateman 2010b).
I am assuming that these and other trends evident in the data gathered for Atlas 2020 will prompt further reappraisals of the conservation threats facing not just orchids but all native components of the British and Irish flora. It is my hope that the new Atlas will also encourage improvements to the way in which those appraisals are conducted. With these goals in mind, I will explore some of the pertinent issues.
IUCN categories: theory
The IUCN have famously developed a sliding scale of eight formal categories of threat (IUCN 2012, 2019): Least Concern (LC), Near Threatened (NT), Vulnerable (VU), Endangered (EN), Critically Endangered (CR), Regionally Extinct (RE: an oxymoron that would more appropriately be replaced by "Extirpated"), Extinct in the Wild (EW) and finally Extinct in the true meaning of the term (EX). Four criteria are employed to assign a species to its most appropriate threat category: degree of decline in area of occupancy (A), reduction in number of locations (B), decline in number of individuals (C), and simply number of individuals irrespective of recent change (D, based on a much lower numerical threshold than C). In practice, criterion A dominates decision-making in the British Isles, and is assessed primarily through increasingly sophisticated statistical methods applied to the hectad records summarised in successive plant atlases spaced approximately 20 years apart. Thus, the series of plant atlases produced for the British Isles, and their underlying databases, have become pivotal to formal conservation assessment of species and subspecies (taxa below the level of subspecies are reliably invisible to conservation protocols). An additional criterion is whether the species is judged convincingly native or is instead relegated to one of the non-native categories.
An equally if not more important distinction lies between species that have already been assigned to a formal threat category versus those that have not. The latter can be designated Data Deficient, placed in the Waiting List, placed in the Parking List, or simply ignored completely (a 'non-status' that is formalised by IUCN using the deceptively anodyne phrase Not Evaluated). The IUCN Red List states that '[A] taxon is Data Deficient (DD) when there is inadequate information to make a direct, or indirect, assessment of its risk of extinction based on its distribution and population status. A taxon in this category may be well studied, and its biology well known, but appropriate data on abundance and/or distribution are lacking.' According to Cheffings et al. (2005: 98), describing the Red List for Great Britain, '[T]here are three main reasons why taxa are placed [in the Waiting List]: (1) inadequate distributional data; (2) taxonomic uncertainties; (3) uncertainty over native, archaeophyte or neophyte status [a pedant might note at this point that neither taxonomic treatment nor native status can ever truthfully be described as "certain"]. A lack of adequate distributional data particularly affects subspecific taxa … This list highlights what research needs to be done in order to clear up population, distributional and taxonomic problems.'
Thus, Data Deficiency appears at first glance to equate with a subset of Waiting List issues relating to (lack of) knowledge of geographic distribution, though one could legitimately argue that optimal taxonomy and accurate assessment of native status are essential pre-requisites for gathering accurate distributional data, in which case the Data Deficiency and Waiting List concepts become fully synonymous. There is an implicit intent that both terms flag up taxa that are in particular need of further (preferably well-funded, professionally led) research, though as noted by Parsons (2016), there is in practice a regrettable tendency for policy-makers to view DD/WL taxa as administrative nuisances and/or as taxa that can comfortably be assumed to be en route to eventually being categorised as Least Concern. Given that less than half of British and Irish orchid species are currently categorised as being of Least Concern, this would be a rash assumption.
Successive British Red Lists have also employed an apparently a uniquely British pigeonhole, the Parking List, for 'taxa rejected from the current analysis, the majority of which have been listed as Red Data Book plants in the past. Reasons for their exclusion include research showing incontrovertible evidence that they should be placed at a lower rank than subspecies … In contrast with the Waiting List taxa, further work on the Parking List plants is not thought to be a priority' (Cheffings et al. 2005: 98). This distinction is clearly of considerable significance, given that the Waiting List is analogous with purgatory whereas the Parking List is analogous with hell as there is no obvious means of escape; it seemingly precludes both resurrection and irrevocable oblivion.
The true importance of taxonomic decisions can now be made explicit. Setting aside the unappealing quagmire of purely nomenclatural issues, taxonomy impacts on our understanding of the all-important geographic distributions in three ways: circumscription (i.e. where are the phenotypic and genotypic boundaries of the taxon?), diagnosability (i.e. how effective are the available phenotypic and genotypic characters at permitting identification of members of the taxon?), and choice of preferred taxonomic rank, which ideally should reflect comprehensive knowledge of both circumscription and diagnosability, considered together within a framework of explicit definitions of specific and infraspecific ranks.
Even given optimal taxonomy, there inevitably ensues a lag phase of at least a decade while field botanists and ecologists become aware of the existence and circumscription of the taxon in question and test its supposed diagnostic characters (sadly, this remains a serious practical challenge in cases where the genotypic characters provide better discrimination than their phenotypic equivalents). In practice, a taxonomic decision made early in one 20-year mapping cycle may not filter through to fieldworkers in time to generate a well-founded map for the atlas that concludes that cycle, emphasising the critical importance of evaluating the rigour of suggested taxonomic changes as quickly as possible and then immediately bringing into play those changes that pass the credibility test. Allowing taxa to wallow for extended periods in the DD/WL categories serves poorly the interests of conservation. We should also note that although the basic unit of taxonomy and evolution is the species, the basic unit of conservation within the British Isles is the subspecies. Consequently, downgrading a taxon from subspecies to variety renders it invisible (or — essentially the same fate — consigns it to the Parking List), yet the crucial conceptual boundary separating the levels of subspecies and variety remains much debated and, as employed by some taxonomists, worryingly interchangeable.
IUCN assessments within the British Isles up to 2006
Table 2 summarises the formal conservation status offered to British and Irish orchid species and subspecies since 1999. The Great Britain Red List underwent major revision in 2005 (Cheffings et al. 2005), in the light of the newly-acquired ability to compare hectad maps in the 1960 and 2000 Atlases, and lesser changes then followed at regular intervals (Leach 2007, 2010, 2017, 2019, 2021; Leach & Walker 2011, 2013, 2015). The GB Red List strongly influenced development of the England Red List (Stroh et al. 2014), while Wales (Dines 2008) and Ireland (Wyse Jackson et al. 2016) — but not Scotland — also developed their own bespoke Red Lists. As noted by Fay (2015), comparison of these lists is instructive, particularly when viewed in the context of the progressive development of the GB Red List.
By the close of the 20th century (Wigginton 1999), the majority of British orchids were still categorised as being of Least Concern. Of the remainder, one was considered Near Threatened (Ophrys sphegodes), four Vulnerable (Orchis simia, O. militaris, Himantoglossum hircinum, Ophrys fuciflora), two Endangered (Liparis loeselii, Orchis purpurea), two Critically Endangered (Cypripedium calceolus, Cephalanthera rubra), and two Regionally Extinct (Epipogium aphyllum, Spiranthes aestivalis). Just six years later, the 2005 Red List (Cheffings et al. 2005) brought about no less than 18 changes of status; two of these species decreased in threat by a single category (Himantoglossum hircinum, Ophrys sphegodes), but the remaining 16 species increased in threat by at least one category. The majority of the species affected by these changes favoured more northerly climates (Fay 2015). Orchis anthropophora and Neotinea ustulata increased in threat by no less than three categories; both leaped in a single bound from Least Concern to Endangered. Particularly unfortunate was the transfer of Epipactis leptochila and E. dunensis from Least Concern to Data Deficient. Presumably, this was the consequence of a decision taken earlier by Richards (1994) to assume conspecificity of these species — a conclusion that has since been definitively disproved through molecular studies (Squirrell et al. 2002; Hollingsworth et al. 2006; Richards & Squirrell 2009; Sramkó et al. 2019; Bateman 2020a).
IUCN assessments within the British Isles: 2006 and beyond
Given that this review focuses on the period immediately following Bateman (2006) and Cheffings et al. (2005), it is changes that follow the 2005 list that are of greatest concern (see also Fay 2015). Subsequent changes in conservation status, made under criteria that remained almost unaltered after 2005, were far more sporadic.
Decisions made in 2015 (Leach & Walker 2015) to downgrade Orchis purpurea to Vulnerable and Himantoglossum hircinum to Least Concern were justifiable, given the under-recording of the former and documented expansion of the latter. And there was little choice but to transfer Epipogium aphyllum from Regionally Extinct to Critically Endangered when a single depauperate plant bearing a single even more depauperate flower was discovered in a Herefordshire wood in 2009 — initially by humans but soon after by a hungry slug (Bateman 2010a; Garner 2010; Jannink & Rich 2010; Cole 2014). Ironically, my 2006 argument (Bateman 2006) that this species should be viewed as Regionally Extinct, and a statistical estimate made in 2009 that its likelihood of having been extirpated was 98.5% (Taylor & Roberts 2011), caused Epipogium to be adopted as the epitome of extirpation by the conservation organisation Plantlife immediately before its rediscovery in Herefordshire. However, since 2009, this infamous 'Lazarus taxon' has not been reported from any of its several former localities in either the Chiltern Hills or the Welsh Borders. I question whether one unhappy plant appearing in only a single summer 12 years ago represents a meaningful resurrection. Given that climatic conditions in Britain are undoubtedly diverging from those preferred by this notoriously unreliable species, it seems unlikely that any last-gasp conservation measures will have succeeded. I would therefore be inclined to immediately return Epipogium aphyllum to the Regionally Extinct category, noting in passing that this most feverishly sought of all British natives is widespread and designated Least Concern at the European level, and hence has a certain future elsewhere.
The piecemeal nature of annual revisions of IUCN status in the British Isles is epitomised by the treatment of the subspecies of Dactylorhiza incarnata (Table 2). Subsp. ochroleuca and subsp. cruenta were relegated to Data Deficient in 2005 but restored to their former categories (Critically Endangered and Endangered, respectively) in 2010 (Leach 2010). In 2013, subsp. ochroleuca was considered for possible elevation to species status (Leach & Walker 2013) whereas subsp. cruenta was not, despite possessing a stronger genetic case (Bateman et al. 2022). Subspecies coccinea escaped from the Waiting List to become of Least Concern (Leach & Walker 2013), but left behind in purgatory its former cell-mate, subsp. pulchella. There could not be a clearer example of why such issues are best tackled through a monographic overview that explicitly integrates all relevant kinds of data (in the case of D. incarnata, this goal would best be pursued through a genetically-informed update of the morphometric monograph provided by Bateman & Denholm 1985).
Arguably the greatest challenges to categorisation are posed by discoveries within the British Isles of taxa previously regarded as exclusively Continental — either because they have only recently been segregated as new taxa, or they have been present in the British Isles for some time but have passed unrecognised, or because they are hypothesised to have recently migrated to Britain and Ireland from the Continent. In such challenging circumstances, four questions relating to taxonomy should always be asked, regarding distinctiveness, taxonomic rank, formal name, and precision of circumscription (Table 3). Two further, non-taxonomic questions then need to be asked. The first of these questions — whether the taxon has been sought with diligence sufficient to acquire sound knowledge of its geographic distribution — typically requires decades to fully answer, and I view it as counter-productive to delay conservation assessments over such periods, particularly in times of such rapid environmental change. But the most challenging question of all to answer, at least for a recently discovered species, is whether the putative immigrant arrived naturally as wind-blown or bird-carried (dust-) seed. The most obvious alternative explanation is that seeds were deliberately spread or tubers deliberately planted, but there is a rapidly increasing probability of an intermediate explanation: that seed originated from nearby cultivated plants, as was suggested for a novel population of Serapias cordigera found in a Kentish quarry in 1996 (Cole & Waller 2020).
Careful observation yields rewards. The extensive dune system at Kenfig in South Wales has been responsible for bringing to our attention no less than three of the problem taxa discussed earlier, initially on the basis of traditional morphological identification: Epipactis helleborine subsp. neerlandica (Lewis & Spencer 2005; Kreutz 2008; Lewis et al. 2009), Gymnadenia conopsea "var. friesica" (Kreutz 2008; Kreutz & Lewis 2015; Lewis 2015) and Dactylorhiza incarnata "subsp. lobelii" (Clark & Lewis 2018). Athough I approached scientific analysis of each of these initial determinations with considerable scepticism, our DNA-based data not only supported the distinctiveness of neerlandica (Sramkó et al. 2019; Bateman 2020a) and friesica but also encouraged raising cf. friesica to a subspecies of G. densiflora (Bateman & Denholm 2019; Bateman et al. 2021b). However, the attribution of this population to the Dutch coastal taxon 'friesica' will remain untested until plants have been subjected to molecular and morphometric analyses in the Frisian Islands; in other words, relative to the questions formalised in Table 3, for this taxon we can answer (1) and (2) but we cannot yet answer questions (3) and (4) [nor (5) and (6)]. Given the surprisingly low degree of genetic differentiation exhibited by Dactylorhiza incarnata, I doubt that "subsp. lobelii" will similarly pass the test of distinctiveness, should suitable tests ever be applied. Subspecies neerlandica was added to the Waiting List in 2011 (Leach & Walker 2011) but subsp. friesica only in 2021 (Leach 2021).
Lastly, we should consider the challenges posed by the two genera that (along with Epipactis helleborine and Anacamptis pyramidalis) are most adept at rapidly occupying recently disturbed soils: Ophrys and Serapias. In 1976, the four long-recognised and fortuitously uncontroversial native British species of the genus Ophrys were joined by a fifth 'microspecies'. Unfortunately, the single plant of O. bertolonii s.l. found in Dorset generated more heat than light (cf. Pankhurst & Matthews 1977; Ettlinger 1990). A subsequent occurrence of O. scolopax found in Somerset in 2011 was eventually revealed to have been deliberately planted. No such admission has yet been issued for plants of putative O. scolopax s.l. found in Dorset (2016) and Cornwall (2021), or for the single plant of O. tenthredinifera s.l. discovered in Dorset in 2014 (Chalk 2014); the fact that, like O. bertolonii, this species is confined to the Mediterranean Basin makes it an unlikely natural immigrant.
Serapias is proving to be an even more tantalisingly mobile genus, given the increasing number of sightings and the fact that members of all three major species groups evident within the genus have now been found in England. Knowledge of the scale of the current climate crisis, and expectation of its ability to modify the distributions of at least some British and Irish orchids, have increased the credibility of recent arrivals as natural occurrences: S. parviflora in Cornwall (1989), Suffolk (2021) and London (2021), S. lingua in Devon (1998) and Essex (2017), S. vomeracea in Kent (2020), and S. cordigera s.l. in the Isle of Wight (1918) and South Yorkshire (2021). The habitats in which these plants were found vary from credibly natural to undeniably synthetic — most notably, a green roof in the case of London's S. parviflora population (topic reviewed by Bateman 2006; Harrap & Harrap 2009; Cole & Waller 2020; Kitchener et al. 2021) — as do the locations of their northern-most outposts within continental Europe (cf. Bournérias & Prat 2005). However, the overall picture of a sharp increase in these novel occurrences during the last quarter century, combined with the documented ability of the genus Serapias to invade new habitats, must surely increase the probability that at least some of these finds arose naturally, their successful establishment presumably aided by climate change.
Unfortunately, it is far easier to use genetic data to identify an orchid than to determine its geographic origin when found in circumstances where it is suspected of being a recent immigrant. An attempt to do so was made by Bateman et al. (2008) for two plants of Orchis purpurea that first appeared at an Oxfordshire site in 1998, where they proceeded to hybridise enthusiastically with the last surviving population of Orchis simia in the Chiltern Hills (discussed above under 'Orchis'). But this effort to at least approximate an ancestral origin relied on already possessing a fortuitous genetic data-set derived from sampled plants scattered across Europe, and such data remain available for only a small minority of British and Irish orchids. Certainly, there are presently no published genetic data-sets capable of providing a framework for genotyping the molecularly recalcitrant Serapias species. In contrast, such analysis might conceivably be performed on any new finds of Cypripedium calceolus that were not part of the widely publicised re-introduction scheme, as this species has through the last quarter-century been subjected to numerous genetic studies. Unfortunately, those studies have been conducted at local or regional scales using contrasting approaches, precluding access to the far more informative bigger picture.
Once again, Red Lists have experienced difficulties categorising these occurrences. Serapias parviflora (in 2005) and S. lingua (in 2019) initially languished on the Waiting List but both were eventually categorised in 2021: lingua as Critically Endangered and parviflora as Regionally Extinct (an adjudication that obviously will require revision in the light of its discovery in London and possibly also Suffolk in 2021). Interestingly, these changes of status were not highlighted by Leach (2021) in his latest IUCN overview. The finds of S. vomeracea in 2020 and of S. cordigera in 2021 have yet to be formally addressed from a conservation perspective (Table 2). It will be interesting to see whether S. cordigera's bid for native status will be penalised for its decision to occupy a suburban lawn, in contrast with the less manicured roadside verge chosen by S. vomeracea.
IUCN assessments within the British Isles: current and future conundra
Thus far, I have focused discussion on IUCN categories for Great Britain, but in this section I will also consider the separate assessments made at national level for England (Stroh et al. 2014), Wales (Dines 2008) and especially Ireland (Wyse Jackson et al. 2016) — a country that has sole responsibility within the British Isles for all surviving populations of Neotinea maculata and Dactylorhiza kerryensis, as well as being particularly rich in Spiranthes romanzoffiana and Dactylorhiza incarnata subsp. cruenta (Table 2).
Happily, the current statuses of those species and subspecies that have been assessed and formally categorised make good sense and require little further consideration. Most of my comments will instead address those taxa that have not yet been categorised, either because they have not been assessed (Data Deficient plus Waiting List), have not been considered for assessment, or have been "de-accessioned" into the decidedly eclectic Parking List.
I will begin by addressing the Great Britain Red List (Cheffings et al. 2005, et seq.), though most of the following comments also apply to the derived England List of Stroh et al. (2014). There is no further justification for deeming Epipactis leptochila and E. dunensis as Data Deficient. Taxonomically, they rank among the most intensively researched British and Irish species of any plant family. They are unequivocally distinct, bona fide species, and on present evidence their distributions are allopatric here, E. leptochila reliably occurring further south than E. dunensis. And although no new data have been gathered on Epipactis 'sancta', subspecies status within E. dunensis is more consistent with improved knowledge of the genetics of E. dunensis and other closely related taxa (Bateman 2020a). Likely outcomes of re-assessment are Least Concern for E. dunensis and Near Threatened or Vulnerable for E. leptochila, which tends to form only small populations and is declining. Admittedly, a rigorous morphometric study is still needed to compare these species as an aid to identification, but this lacuna is far from sufficient justification for their ongoing categorisation as Data Deficient.
The molecular work of Sramkó et al. (2019) clearly undermines the argument of Kreutz et al. (2020) that a small population of Epipactis in the Chiltern Hills should form the basis of a new variety to E. neglecta, a supposed species formerly regarded as exclusively Continental. DNA data make clear that this population is far more realistically viewed as representing introgression between E. helleborine and E. leptochila, as well as demonstrating that E. 'neglecta' is in fact nothing more than a synonym of E. leptochila (Bateman 2021c).
DNA data also unequivocally demonstrate that Epipactis helleborine subsp. neerlandica occurs in South Wales, is best treated as a subspecies, and has been correctly named — it is the same taxonomic entity as that characteristic of coastal dunes in the Netherlands and Denmark (cf. Jacquemyn et al. 2020). The relative obscurity of this taxon, and its choice of a highly appropriate habitat, suggest that it is likely to be a natural long-distance immigrant. What is needed at this point is a search for other British and Irish populations of this subspecies in order to clarify its distribution, but I do not believe that this gap in our knowledge offers sufficient justification for leaving it on the self-defeating Waiting List for a further decade. This subspecies has generated a substantial metapopulation in South Wales, implying designation as Vulnerable on both the Great Britain and Wales lists.
The rarity of Epipactis leptochila and E. atrorubens in Wales is well-reflected in the Welsh Red List, but the evidence that E. leptochila has ever occurred in Ireland is unconvincing; it does not merit its current place on the Ireland Waiting List. On the other hand, the discovery of small numbers of E. dunensis near Dublin in 2019 (Santos & Sayers 2021), albeit still requiring molecular confirmation, should encourage botanists to seek further populations of this species in suitable habitats in Ireland. On present evidence, the clusters of small numbers of plants thus far detected, all occupying unstable habitats, would suggest a designation of Critically Endangered. In contrast, sufficient scattered populations of E. phyllanthes have been found in Ireland since Atlas 2000 (Stroh et al. 2022) to perhaps justify reduction in its Irish status from Endangered to Vulnerable. And the fact that E. phyllanthes has been shown by Sramkó et al. (2019) to encompass several supposed species of central and eastern Europe and Asia Minor, including E. 'exilis' and E. 'persica', means that it need no longer be viewed as an "international responsibility" of Britain, as we can no longer be "sure that the UK holds more than 25% of European populations" (Stroh et al. 2014: 23); indeed, this claim now appears unlikely to be true.
The Welsh Red List will need to assess the significance of the first Welsh occurrence of Spiranthes romanzoffiana, a small but vigorous population found in 2019 north of Aberystwyth (reported in the 2020 addendum to Parker 2016) (Fig. 6E). This discovery partially compensates for the extirpation of this species from England (it was recorded on Dartmoor between 1957 and 1994). Elsewhere in England, populations of Goodyera repens formerly scattered across Norfolk have declined to perhaps only one small coastal population (Stroh et al. 2022), placing greater reliance on the less climatically vulnerable populations in Cumbria and Northumbria. It might therefore be wise to formally re-assess this species, which may now justify Vulnerable status in England.
In 2005, the GB Red List elevated Platanthera bifolia from its former status of Least Concern to Vulnerable, following concerns raised by its apparently substantial decline in the 2002 Atlas (Preston et al. 2002) that inspired a subsequent citizen science 'Back from the Brink'-style mapping exercise coordinated by Plantlife. Moreover, the 2014 England Red List regarded this species as Endangered. Although P. bifolia is undoubtedly declining in England, its recently conferred Endangered status may represent an over-reaction to that decline. However, it can also be viewed as presaging an urgent need to re-examine the genetics of British and Irish Platanthera using next-generation techniques. The relevant ecotypes (discussed above under 'Platanthera') are not discriminated in Atlas 2020. My anecdotal field experiences suggest that P. bifolia s.s. is holding reasonably firm in its acid heathland habitats, but that the woodland ecotype in particular has declined considerably in recent decades. If the woodland ecotype does prove to be congruent with the Continental P. fornicata and/or P. muelleri, it will become a more serious conservation concern and will likely then earn its present rating of Endangered.
The current designations within Gymnadenia require swift revision. At present, G. conopsea and G. borealis are correctly designated Least Concern, whereas G. densiflora has been considered Data Deficient since 2005 (Table 2). This situation makes little sense. From the viewpoint of mapping through morphological identification, the main challenge is to separate the more morphologically distinct G. borealis and G. densiflora from G. conopsea s.s., which has a largely intermediate phenotype (Bateman & Denholm 2019; Bateman et al. 2021b). Gymnadenia conopsea s.s. is effectively the residue after G. borealis, G. densiflora subsp. densiflora and, most challengingly, G. densiflora subsp. cf. friesica have been subtracted. So in practice, it is G. conopsea s.s. that is most deficient in data at present, rather than G. densiflora. With regard to the seven key questions outlined in Table 3, only question (5) remains seriously problematic. The distribution maps given in Atlas 2020 offer much stronger patterns than the equivalent maps in Atlas 2000, sketching a credible picture of G. conopsea s.s. dominating limestone soils and hence being concentrated toward the south, G. densiflora having a scattered distribution across the British Isles in moist alkaline soils, and G. borealis occupying heathland habitats and thus being dominant in Scotland. But G. conopsea s.s. is shown in Atlas 2020 as occupying several hectads in Scotland when, in truth, we do not yet have conclusive evidence that G. conopsea s.s. even occurs in Scotland. Obviously, decades of further fieldwork will be needed to clarify fully the distributions of the three species, but this realisation is not sufficient reason for retaining Data Deficient status — awarding a provisional designation (of Least Concern for all three Gymnadenia species) makes far greater practical sense.
Admittedly, formally categorising Gymnadenia densiflora subsp. cf. friesica will be more challenging. When subjected to the questions listed in Table 3, this taxon is demonstrably barely distinguishable using morphological criteria, has probably but not definitely been assigned to the most appropriate taxonomic rank, may or may not bear the most appropriate formal epithet, presently has a questionable circumscription in need of further testing, and so understandably has a poorly understood geographic distribution. This taxon seems most likely to have immigrated into Britain naturally, but this assumption cannot be proven. Given these ambiguous properties, it may be no surprise that cf. friesica was placed on the Waiting List in 2021 (Leach 2021). Yet just what exactly are we waiting for? Given that it is apparently a rare taxon occupying biodiverse yet vulnerable habitats (Bateman et al. 2021b), are we not better served by awarding this subspecies a provisional designation now (of Vulnerable, based on present evidence), rather than succumbing to caution by placing it in conservational limbo? These decisions impact on both the Wales and England Red Lists as well as the GB List.
The piecemeal treatment of the subspecies of Dactylorhiza incarnata (discussed above) would best be remedied by continuing to base assessments on the taxonomic treatment provided by Bateman & Denholm (1985), at least until the subspecies have been further tested through comparison using high-throughput sequencing and/or more intensively sampled morphometric analyses. As the circumscription of subsp. pulchella is actually no more contentious than those of any other subspecies, it should be immediately rescued from the Waiting List and designated Least Concern. In contrast, serious challenges to the taxonomic credibility of subsp. gemmana (including unpublished morphometric data of Bateman & Denholm) suggest that, at best, it does indeed remain Data Deficient.
Among the tetraploid marsh-orchids, only the Irish endemic Dactylorhiza kerryensis is presently deemed Data Deficient. In fact, this species definitively passes all the tests listed in Table 3, with the arguable exception of having an imperfectly known distribution. In Ireland, an already complex admixture of Dactylorhiza species has been further complicated by some counter-productively individualistic taxonomic treatments (e.g. Curtis & Thompson 2009); it therefore proved necessary to critically appraise individual records when preparing the relevant distribution maps for Atlas 2020. Nonetheless, the map for D. kerryensis clearly demonstrates a preference for the Atlantic coastal regions, where its broad habitat tolerance means that D. kerryensis is frequent and is best designated Least Concern. Indeed, in Ireland, this species is arguably more accurately mapped than D. purpurella, with which it is often confused. Dactylorhiza traunsteinerioides, a species with an Irish holotype, is becoming more accurately mapped in Ireland. Nonetheless, a watching brief should be placed on the ongoing possibility that rare anthocyanin-rich populations of D. traunsteinerioides in Co. Antrim (e.g. Ennis 2007) may be better ascribed to subsp. francis-drucei, unequivocally located only 40 km distant in southwest Scotland. Lastly, it would not be surprising if D. praetermissa proved to have recently migrated to Ireland, given its current inexorable northward march across the British Isles and its newly-acquired dominance along the seaboards of Wales and Lancashire (Fig. 9).
One notable and inexplicable omission from both the GB and England Red Lists is Dactylorhiza praetermissa subsp. schoenophila, established a decade ago by Bateman & Denholm (2012) to accommodate fen-dwelling populations of D. praetermissa is southern England that were formerly misidentified as belonging within D. traunsteinerioides. At present, the main uncertainty regarding this taxon is the extent of its distribution — in particular whether it occurs north of a line linking the Severn Estuary to the Wash (Bateman 2020b) — but in my opinion this lacuna should be insufficient to preclude provisional GB Red-Listing, most likely as Near-Threatened.
A peculiar smorgasbord of supposed dactylorchid subspecies currently populate the Waiting List, all of which are either better treated as varieties or simply consigned to history; in either case their claims to participate in the Red-Listing process are weak. Dactylorhiza purpurella var. cambrensis even has the dubious privilege of concurrently appearing on the Parking List, in its alternative guise of D. purpurella subsp. majaliformis. Other taxa affected are a host of spotted-orchid variants that have been repeatedly rejected as biologically meaningful following scientific study (Bateman & Denholm 1989, 2003; Pillon et al. 2007; Bateman 2021b) and whose sole unifying property appears to be passing mention in the text of Stace (2010, 2019).
Lastly, we should address what appears to be a concerted effort by the genus Serapias to invade the British Isles. Serapias parviflora was relegated to Regionally Extinct in 2021, the same year that it was found on top of a skyscraper in London and so rendered a candidate for Critically Endangered status, where it could legitimately be joined by two other recent arrivals, S. vomeracea and S. cordigera (see 'Serapias' above). Unless one chooses to dabble in the numerous microspecies recognised by some Mediterranean orchid specialists (e.g. Delforge 2016), these particular Serapias species are not taxonomically problematic (i.e. questions (1) – (5) in Table 3). Rather, they raise questions that are very difficult to answer with confidence, concerning how they arrived at their current locations and where they originated (i.e. questions (6) and (7) in Table 3). Precisely the same challenge is presented by the Oxfordshire locality for Himantoglossum robertianum.
We can usefully conclude this section by identifying additional orchid species that might soon succeed in migrating northward across the English Channel. Parsing through orchid floras of France (Bournérias & Prat 2005) and the Low Countries (Kreutz 2019) revealed relatively few remaining taxa that are well-placed geographically to attempt the crossing. Any migrating Dactylorhiza majalis and D. sphagnicola that succeeded in reaching maturity in the British Isles would probably rapidly lose their genetic integrity as a consequence of immediately experiencing gene-flow with larger numbers of endemic Dactylorhiza species already present. Careful study would be needed to distinguish Epipactis muelleri from E. leptochila and from some of the diverse phenotypes evident within E. helleborine. Epipactis microphylla and the obligate mycoheterotroph Limorodum abortivum would be easier to identify, as would Anacamptis laxiflora, which has already formed substantial populations on the Channel Islands (e.g. Wood & Ramsay 2004). Although climate change might be considered to positively encourage invasions of further Ophrys species, the only non-British taxa currently well-placed to cross the Channel (the microspecies O. "aranifera" and O. "araneola") are actually only minor phenotypic variants that are already represented within, and are genetically indistinguishable from, our existing native populations of O. sphegodes (Bateman et al. 2021a). Global warming decreases the probability of Spiranthes aestivalis returning to our shores across the Channel following its extirpation from the New Forest in the 1950s, and the combination of warming climate and prevailing westerly winds reduces the likelihood of cool-loving Scandinavian natives such as Pseudorchis straminea, Chamorchis alpina and Calypso bulbosa becoming established in the British Isles.
IUCN assessments in a European and global context
The conservation categorisations of orchids summarised by Bilz et al. (2011) on a European scale appear to have been accepted without challenge when being incorporated into the global Red List (IUCN 2021), so the two scales can be addressed together here. With regard to uncategorised taxa, two damaging decisions become apparent. Firstly, at these broader geographic scales, subspecies join varieties and formae in becoming invisible to conservation policy, to the detriment of British and Irish Dactylorhizas in particular. Secondly, the genus Dactylorhiza suffers the further indignity of having our four tetraploid marsh-orchids amalgamated into an aggregate D. majalis. This decision ignores several key facts; these species can be distinguished reliably using both genotype (Pillon et al. 2007; Brandrud et al. 2020) and phenotype (Bateman & Denholm 1983, 2012) and also have evolutionary origins separate from that of D. majalis, which does not occur in the British Isles (Hedrén et al. 2008; Brandrud et al. 2020). Recognition of an exceptionally broad D. majalis also fails to acknowledge that most Continental orchid monographers based outside Scandinavia treat the British and Irish allotetraploid taxa as full species (e.g. Delforge 2016; Eccarius 2016; contra Kühn et al. 2019).
These turbulent waters are further muddied by an obvious logical inconsistency. Gymnadenia borealis, raised to species status only following molecular analysis in 1997, is categorised as Data Deficient (Rankou 2011), whereas G. densiflora remains unassessed, despite being equally distinct both morphologically (Bateman et al. 2021b) and molecularly (Bateman et al. 2018a; Brandrud et al. 2019) and being more widely recognised taxonomically than G. borealis throughout Europe (albeit rarely at species level). I presume that Epipactis dunensis is labelled Data Deficient because it was temporarily confused with E. leptochila in Britain during the 1990s. A better reason for revised assessment was provided by the RAD-seq study of Brandrud et al. (2020), which showed that the Continental 'E. bugacensis' and 'E. rhodanensis' are actually synonymous with E. dunensis, thereby greatly extending the distribution of this species from being a (?near-)British endemic to stretching eastward across Europe at least as far as Hungary (Bateman 2020a). Assigning Data Deficient status to Spiranthes aestivalis and Herminium monorchis — the first species extirpated from Britain, and the second species rapidly declining here — was justified in the European Red List on the highly questionable basis of inadequate knowledge of the distributions of these species in eastern Europe. This reticence ignores the fact that enough knowledge is available in western Europe to be aware that both these species are under threat, as well as failing to acknowledge that excellent orchid floras are now being generated in eastern European countries such as Hungary (Molnár 2011).
The net result of these suboptimal decisions is that the few orchidological crown jewels that the British Isles possess are hidden from view at the European level, including all three potential endemics (Dactylorhiza kerryensis, D. traunsteinerioides, Gymnadenia borealis). Interestingly, all three of these species have dominantly north-westerly distributions occupying formerly glaciated terrains (indeed, a broad-brush survey of subfamily Orchidoideae has rather boldly concluded that cooling climates accelerate speciation rates: Thompson et al. 2021). Of those British and Irish species that have been formally assessed at a European level, most are categorised as Least Concern, and just four of our acknowledged species achieve the rarefied heights of Near-Threatened: Cypripedium calceolus, Liparis loeselii, Spiranthes romanzoffiana and Anacamptis morio (Table 2). Although, as discussed above, tweaks of status are needed at the national and regional scales, it is categorisation at the European scale that is most in need of serious reappraisal. Although the timing may be suboptimal from a political viewpoint, there is a clear and urgent need to renegotiate the official status of British and Irish orchids on the European stage.
Recommendations: Red Lists
By now, it will be clear to the reader that I believe that the Red Listing process should be reappraised. I find no obvious fault in the current IUCN criteria, which are capable of yielding sensible results that achieve a good balance between current status and projected change. Rather, my concerns focus on what I view as the unnecessary and ambiguous hurdles that a putative species or subspecies is required to overcome before it becomes entitled to formal categorisation.
At present, there is no obvious pattern that can be discerned in the GB Red List from either the range of taxa viewed as Data Deficient, the range of taxa allocated to the Waiting List, or taxa that have qualified for neither the limbo of the Waiting List or the purgatory of Data Deficiency. At present, taxa deemed Data Deficient are among those that have been subjected to recent, high-profile research affecting the genera Epipactis, Gymnadenia and Dactylorhiza. This outcome seems a little perverse, given that most of the orchid species that have been assigned to IUCN categories have in fact been plucked off the shelf of alpha-taxonomy, rather than having undergone any serious scientific exploration. The Waiting List consists mostly of Dactylorhiza taxa that are referred to, albeit only in passing, as questionable subspecies by Stace (2010, 2019), despite the fact that earlier specialist studies had concluded that the taxa in question were better viewed as varieties (Bateman & Denholm 1989, 2012). These taxa appear alongside three putative orchid subspecies whose main claim to fame is having been featured in BSBI News (Lewis et al. 2009; Clark & Lewis 2018; Bateman & Denholm 2019). However, two new subspecies of Dactylorhiza that were published in BSBI's then scientific journal New Journal of Botany a decade ago by Bateman & Denholm (2012) have not yet qualified for even the Waiting List. Perhaps the most impressive juxtaposition of dates is the release of a UK Biodiversity Action Plan for the biologically valueless Epipactis 'youngiana' in 2007 (Anonymous 2007), ten years after its value had first been seriously questioned (Harris & Abbott 1997) and one year after it had been unequivocally unmasked as a minor variant of E. helleborine (Hollingsworth et al. 2006). The Parking List is again dominated by Dactylorhiza, but in this case the names listed are discredited synonyms, most of which appeared in early editions of Stace's (1991, 1997) flora and the competing traditional flora of Sell & Murrell (1996). There is nothing special about these synonyms when they are viewed in the context of the plethora of other synonyms that perennially handicap investigations of European orchids.
Indeed, seen from a European perspective, the names residing under Data Deficient or patiently populating the Waiting List appear remarkably insular. Continental orchid enthusiasts maintain a veritable production line of new orchid taxa, most of which are published in relatively obscure non-electronic journals and magazines specialising in European orchids. Admittedly, very few of these taxa are underpinned by any attempt at scientific analysis and it is questionable whether most would survive serious scrutiny. Most of the minority of novel taxa that are awarded species or subspecies status rather than variety or forma reflect a recent trend towards atomised taxonomy that is impossible to justify adequately on biological grounds. This phenomenon adversely affects genera such as Serapias, Gymnadenia subgenus Nigritella, Epipactis section Epipactis, and especially Ophrys. Once created, these numerous microspecies cause further damage by creating the potential, through supposed hybridisation, for establishing far greater numbers of formally named 'nothospecies' and 'nothosubspecies'. Critically appraising these perennially irritating taxa is unappealing but unfortunately it is difficult to justify wholly ignoring them. In practice, most can be side-lined immediately by simply asking the pragmatic question of whether the names are underpinned by any scientific data.
I suggest that the approach taken to future decision making (for all vascular plants, not just orchids) should be even more explicitly evidence-based — a goal to be achieved by applying in sequence the seven questions outlined in Table 3. The Parking List should be abandoned and the Data Deficient category — which leaves the affected taxa too easily ignored or viewed as a de facto Least Concern judgement (e.g. Parsons 2016) — should be retired, thus leaving only the Waiting List. The Waiting List should then be reconfigured to include only taxa for which the answer to Question 1 (diagnosability) is 'Yes' but the answer to Question 2 (most appropriate rank) is 'We don't yet know'. Any taxon supported by data sufficient to permit an answer of 'Yes' to Question 2 should immediately be scheduled for formal categorisation. A particularly useful innovation would be to introduce parenthetic suffixed notes to Red List assessments that signify whether the answers remain equivocal to Question 3 (nomenclature), Question 4 (circumscription) and/or Question 5 (distribution).
The approach recommended here would simplify the present formal categorisations, reduce the frequency of uncategorised taxa, and make more explicit any lacunae remaining in the relevant knowledge base, potentially stimulating research to fill those lacunae. Most importantly, it would also eliminate the present criminal injustice of taxa being refused categorisation because they have been subject to research that failed to fully answer all seven key questions. It is essential to comprehend that the majority of species that superficially appear unproblematic do so simply because scientific questions have either never been asked of them or have been asked only in a counter-productively parochial UK context. It is particularly important that formal categorisation of threat is not held back by Question 5 (i.e. by the British and Irish Plant Atlas itself). Although there exists a crucial feedback loop between taxonomic classifications, identification aids and species distributions, the 60-year sequence of BSBI Atlases has demonstrated that this process inevitably incurs a long inbuilt delay — a delay too protracted to be allowed to continue hindering crucial threat assessments.
Provisional categorisation is not in practice a novel suggestion; the history of the Great Britain Red List in particular demonstrates admirable willingness to modify categorisations as relevant data accumulate. Obviously, the suggested approach to accelerating IUCN categorisation would require the relevant literature to be carefully monitored and any novel taxa and novel immigrants rapidly triaged to weed out the undeserving majority — taxa whose claim to legitimacy rests entirely on the traditional, non-analytical, pre-scientific taxonomic methods of the 18th century.
Recommendations: Future research
It is unfortunate that the study of the British and Irish flora has become not only almost wilfully insular but also dangerously unambitious. Remarkably few of the more heavyweight publications cited in the present review were produced by UK-based institutions. The long-term mapping programme that generates successive Atlases of Britain and Ireland remains the jewel in the crown, but it is symptomatic that of 124 publications listed as supporting the latest Great Britain Red List, only 11 were published in Scopus-registered outlets (and of these, only four were published during the last decade). Scopus registration is, unfortunately, a basic academic requirement that, within the context of the Botanical Society of Britain and Ireland, has not been achieved by its successive journals Watsonia, New Journal of Botany or British & Irish Botany. The remaining 113 publications would, for example, be invisible to the Research Excellence Framework — the yardstick by which the careers of Britain's university academics are presently forcibly measured. This difficult situation has not been improved by the imposition of wastefully punitive open access fees for mainstream scientific publication; much of what little data are generated consequently become entombed in grey literature and unpublished reports. It is therefore unsurprising that systematic botany has been almost eliminated from both the British and Irish university sectors. In addition, the last bastions of the discipline — national botanic gardens and museums — are now under threat, as attempting to remedy humankind's ever-increasing impact on the environment is given political precedence over further refining the systematic biology of the European flora.
The British Isles are indeed islands and have been so for much of the last 100,000 years, even if a temporary land-bridge to the Low Countries existed as little as 8,000 years ago. Consequently, our orchid flora was compared with those of many other archipelagos globally in one recent study (Taylor et al. 2019), rather than with that of mainland Europe. It is likely that the flora of the British Isles largely accumulated since the tundra-like conditions of the Younger Dryas interstadial period ended approximately 11,600 calendar years ago, thereby explaining our relatively impoverished biodiversity per unit area. There has since been sufficient time for only very few credible speciation events to have occurred within Britain and Ireland. Of the 53 orchid species now considered probably native to the British Isles, only Gymnadenia borealis plus two or at most three (near-) endemic and hybridogenic tetraploid Dactylorhizas offer the serious possibility of having speciated here, and all are only subtly distinct from their closest relatives occupying mainland Europe.
In truth, our flora makes sense only when placed in the broader geographic context of where these species, or their recent ancestors, originated. Most of our orchids are immigrants that undoubtedly arrived from the south and east — that is, from continental Europe — the only obvious exception being the amphi-Atlantic species Spiranthes romanzoffiana (Forrest et al. 2004). A question asked less often is whether some orchid species may have emigrated outwards from the British Isles, a question encouraged by the very limited occurrence of Dactylorhiza purpurella along the North Sea seaboard of continental Europe and the Faroe Islands (e.g. Pedersen 2007; Eccarius 2016), and the possible occurrence of populations of D. traunsteinerioides (Bateman 2019) and Gymnadenia borealis (Bjerke & Strann 2009; Bateman et al. 2018a) along the Norwegian coast.
Indeed, it was the ground-breaking dot-mapped atlas of the Scandinavian flora (Hultén 1950) that precipitated production of the first hectad-based plant atlas of Britain and Ireland (Perring & Walters 1962). Max Walters, co-author of the first British and Irish Atlas, later published the text of a public address that emphasised the importance of a European context for studies of the British flora, and would function equally well as a mission statement for the present work. He argued that "the flora of Britain is almost wholly an outpost of the larger European flora; … there is reason to believe that our native flora has attained its present range in the country as a result of past migrations brought about largely by climatic change", also noting that "a European view of the British flora inevitably alters our priorities, not least in the very urgent and practical questions of nature conservation" (Walters 1984: 5 – 6).
Compared with the orchid flora of continental Europe, we have a higher ratio of genera to species. It is no coincidence that Part 1 of the present text focuses on genera that are relatively diverse taxonomically in the British Isles, where they have attracted considerable scientific attention. However, it is important to appreciate that species representing the majority of our native orchid genera are mere recently-arrived ambassadors of groups that are far more diverse elsewhere. The fact that these ambassadors are readily identified using their morphology lulls us into a false sense of security that they are well-understood and can safely be regarded as unequivocally circumscribed, perfectly morphologically characterised and correctly named. Nothing could be further from the truth; they are actually likely to have been subjected to even less serious research and to have been even less effectively circumscribed. Genera widely distributed in the Northern Hemisphere and still in need of more thorough integrated monography include Spiranthes (Dueck et al. 2014), Herminium (Raskoti et al. 2017) and Liparis (Efimov et al. 2021). Viewed from a broader geographic perspective, such genera are in fact no less 'critical' than Epipactis or Dactylorhiza, and re-circumscription could easily alter how we perceive the few British species that belong to these genera.
Studies confined to the British Isles can ask only a very limited range of questions. There is no question that Britain excels in generating distributional data on our flora (Stroh et al. 2022) and, more recently, collating distributions with several other sources of data (Henniges et al. 2022). Also, the recently initiated 'Darwin Tree of Life' project aims to apply next-generation sequencing to at least one plant of every putative plant species native to the British Isles (at the time of writing, of the 53 putatively native orchid species listed in Fig. 1, only Dactylorhiza fuchsii, Cephalanthera damasonium and Anacamptis pyramidalis have been scheduled for analysis: darwintreeoflife.org). But data collation and typological analyses cannot address the issue of taxonomic circumscription that is inherent to questions (1) to (3) and especially (4) in Table 3 (Bateman 2011b, 2022), and without which conservation categorisation becomes little more than entrail-spreading.
I have long argued that major progress in British and Irish biosystematics will be achieved only when DNA-based technology is placed in the hands of field biologists (e.g. Bateman 2016). Sadly, a combination of lack of resources and lack of determination has thus far left the affordable, mobile, GPS-style DNA sequencer languishing in the realm of science fiction. However, an alternative, more pragmatic approach would be to establish and fund a DNA facility explicitly designed and run to sequence well-documented samples, accompanied by appropriate documentation, submitted through the mail by British and Irish field botanists. This organisation would initially need to operate through relatively old 'barcode' style sequencing if it were to adequately exploit the pre-existing public databases, but eventually sufficient reference data should accumulate to facilitate more sophisticated data-generating techniques. Such a system would allow better circumscription of known existing taxa, reveal new 'cryptic' taxa, and identify with confidence not only the parentage of putative hybrids but distinguish 'mother' from 'father', as well as hinting at the place of origin of any newly found putative immigrant.
Other — perhaps strongly contrasting — visions may well exist of how research on the British and Irish flora could be revitalised; if so, there is an urgent need that they are openly articulated for debate. The one certainty is that, until research on the British and Irish flora is taken more seriously, we will not be able to produce optimal IUCN assessments of our native flora. Nor will we be well-placed to predict how it will respond to the many threats to which it is increasingly being subjected, or to attempt to at least partly mitigate those threats. I find the British and Irish botanical community to have become increasingly pervaded by a damaging assumption that, from a biosystematic perspective, the flora of the British Isles has been "completed". Thus, field botanists can happily pigeon-hole their finds into taxa competently summarised in Stace (2019), while data analysts can employ tools such as successive Atlases (Preston et al. 2002; Stroh et al. 2022) to better understand the ecology of those putative species. Such studies are essential to fulfil the ambition of predicting the future behaviour of our native plants in the face of environmental challenges such as anthropogenic climate change. Both of these widely admired works are remarkable achievements. But both field botanists and ecologists — and even increasingly systematic botanists — seem to remain worryingly untroubled by the notion that they are undoubtedly still employing as basic units of biodiversity many named taxa that lack biological reality.
References
Adams, K. (2018). A Greater Tongue-orchid (Serapias lingua) colony discovered at Tiptree. Essex Bot. Newsl. 6: 3 – 4.
Ambroise, V., Esposito, F., Scopece, G. & Tyteca, D. (2020). Can phenotypic selection on floral traits explain the presence of enigmatic intermediate individuals in sympatric populations of Platanthera bifolia and P. chlorantha (Orchidaceae)? Pl. Spec. Biol. 35: 59 – 71.
Anonymous (2007). Report on the Species and Habitat Review. Joint Nature Conservation Committee, Peterborough.
Antonetti, M., Nin, S., Burchi, G., Biricolti, S. & Gori, M. (2021). Himantoglossum adriaticum H. Baumann × Himantoglossum robertianum (Loisel.) P. Delforge: a new interspecific hybrid assessed by barcoding analysis. Plants 10: 107 [19 pp.]
Ardelean, C., Ardelean, A. & Boceanu, B. (2018). New Epipactis (Orchidaceae) species for the Romanian flora. Res. J. Agric. Sci. 50: 3 – 15.
Ayasse, M., Gögler, J. & Stökl, J. (2010). Pollinator-driven speciation in sexually deceptive orchids of the genus Ophrys. In: M. Glaubrecht (ed.), Evolution in action, pp. 101 – 118. Springer, Berlin.
Baguette, M., Bertrand, J. A. M., Stevens, V. M. & Schatz, B. (2020). Why are there so many bee orchid species? Adaptive radiation by intra-specific competition for mnesic pollinators. Biol. Rev. 95: 1630 – 1663.
Bailarote, B. C., Lievens, B. & Jacquemyn, H. (2012). Does mycorrhizal specificity affect orchid decline and rarity? Amer. J. Bot. 99: 1655 – 1665.
Balao, F., Tannhäuser, M., Lorenzo, M. T., Hedrén, M. & Paun, O. (2016). Genetic differentiation and admixture between sibling allopolyploids in the Dactylorhiza majalis complex. Heredity 116: 351 – 361.
____, Trucchi, E., Wolfe, T. M., Hao, B.-H., Lorenzo, M. T., Baar, J., Sedman, L., Kosiol, C., Amman, F., Chase, M. W., Hedrén, M. & Paun, M. (2017). Adaptive sequence evolution is driven by biotic stress in a pair of orchid species (Dactylorhiza) with distinct ecological optima. Molec. Ecol. 26: 3649 – 3662.
Bateman, R. M. (2006). How many orchid species are currently native to the British Isles? In: J. Bailey & G. Ellis (eds), Current taxonomic research on the British & European flora, pp. 89 – 110. Botanical Society of the British Isles, London.
____ (2009). Evolutionary classification of European orchids: the crucial importance of maximising explicit evidence and minimising authoritarian speculation. J. Eur. Orch. 41: 243 – 318.
____ (2010a). The Ghost Orchid vividly illustrates why the term ‘extirpation’ is an essential element of plant conservation. BSBI News #113: 8 – 10.
____ (2010b). Where does orchid conservation end and gardening begin? J. Hardy Orch. Soc. 7 (4): 119 – 133.
____ (2011a). Glacial progress: do we finally understand the narrow-leaved marsh-orchids? New J. Bot. 1: 2 – 15.
____ (2011b). The perils of addressing long-term challenges in a short-term world: making descriptive taxonomy predictive. In: T. R. Hodkinson, M. B. Jones, S. Waldren & J. A. N. Parnell (eds), Climate change, ecology and systematics, pp. 67 – 95. Systematics Association Special Volume 78. Cambridge University Press, Cambridge.
____ (2012a). Circumscribing genera in the European orchid flora: a subjective critique of recent contributions. Ber. Arbeitskr. Heim. Orch. 29: 92 – 124.
____ (2012b). Circumscribing species in the European orchid flora: multiple datasets interpreted in the context of speciation mechanisms. Ber. Arbeitskr. Heim. Orch. 29: 160 – 212.
____ (2014). Plumbing the depths: a review of recent research on terrestrial orchid mycorrhizae. J. Hardy Orch. Soc. 11 (3): 81 – 93.
____ (2016). Après le déluge: ubiquitous field barcoding should drive 21st century taxonomy (Ch. 6). In: P. D. Olson, J. Hughes & J. A. Cotton (eds), Next generation systematics, pp. 123 – 153. Cambridge University Press, Cambridge.
____ (2018). Two bees or not two bees? An overview of Ophrys systematics. Ber. Arbeitskr. Heim. Orch. 35: 5 – 46.
____ (2019). Next-generation dactylorchids. J. Hardy Orch. Soc. 16 (4): 114 – 128.
____ (2020a). Implications of next-generation sequencing for the systematics and evolution of the terrestrial orchid genus Epipactis, with particular reference to the British Isles. Kew Bull. 75: 4 [22 pp.]
____ (2020b). False Pugsley's Marsh-orchid. J. Hardy Orch. Soc. 17 (3): 87 – 97.
____ (2021a). Phenotypic versus genotypic disparity in the Eurasian orchid genus Gymnadenia: exploring the limits of phylogeny reconstruction. Syst. Biodivers. 19: 400 – 422.
____ (2021b). Challenges of applying monophyly in the phylogenetic shallows: taxonomic reappraisal of the Dactylorhiza maculata group. Kew Bull. 76: 675 – 704.
____ (2021c). Epipactis 'neglecta var. collina' fails the tests of biological cohesion and basic logic. Ber. Arbeitskr. Heim. Orchid. 38: 100 – 110.
____ (2022). Species circumscription within 'cryptic' clades: a nihilist's view. In: A. Monro & S. Mayo (eds), Cryptic species, pp. 36 – 77. Systematics Association Special Volume 89. Cambridge University Press, Cambridge. [in press]
____ & Denholm, I. (1983). A reappraisal of the British and Irish dactylorchids, 1. The tetraploid marsh-orchids. Watsonia 14: 347 – 376.
____ & ____(1985). A reappraisal of the British and Irish dactylorchids, 2. The diploid marsh-orchids. Watsonia 15: 321 – 355.
____ & ____(1989). A reappraisal of the British and Irish dactylorchids, 3. The spotted-orchids. Watsonia 17: 319 – 349.
____ & ____(2003). The Heath Spotted-orchid (Dactylorhiza maculata (L.) Soó) in the British Isles: a cautionary case-study in delimitating infraspecific taxa and inferring their evolutionary relationships. J. Eur. Orch. 35: 3 – 36.
____ & ____(2012). Taxonomic reassessment of the British and Irish tetraploid marsh-orchids. New J. Bot. 2: 37 – 55.
____ & ____(2019). Mapping the near-cryptic Fragrant Orchids of Britain and Ireland. BSBI News #140: 6 – 12.
____, ____, McLeod, L., Craig, W. & Ennos, R. E. (2022, in review). Systematic reappraisal of Marsh-orchids native to Scotland. Kew Bull. 77.
____ & Hollingsworth, P. M. (2004). Morphological and molecular investigation of the parentage and maternity of Anacamptis ×albuferensis (A. fragrans × A. robusta), a new hybrid orchid from Mallorca, Spain. Taxon 53: 43 – 54.
____, ____, Preston, J., Luo, Y.-B., Pridgeon, A. M. & Chase M. W. (2003). Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot. J. Linn. Soc. 142: 1 – 40.
____, ____, Squirrell, J. & Hollingsworth, M. L. (2005). Tribe Neottieae: Phylogenetics. In: A. M. Pridgeon, P. J. Cribb, M. W. Chase & F. N. Rasmussen (eds), Genera Orchidacearum 4. Epidendroideae 1, pp. 487 – 495. Oxford University Press, Oxford.
____, James, K. E. & Rudall, P. J. (2012). Contrast in levels of morphological versus molecular divergence between closely related Eurasian species of Platanthera (Orchidaceae) suggests recent evolution with a strong allometric element. New J. Bot. 2: 110 – 148.
____, Molnár, A. V. & Sramkó, G. (2017b). In situ morphometric survey elucidates the evolutionary systematics of the Eurasian Himantoglossum clade (Orchidaceae, Orchidinae). PeerJ 5: e2893.
____, Murphy, A. R. M., Hollingsworth, P. M., Hart, M. L., Denholm, I. & Rudall, P. J. (2018a). Molecular and morphological phylogenetics of the digitate-tubered clade within subtribe Orchidinae s.s. (Orchidaceae: Orchidinae). Kew Bull. 73: 54 [30 pp.]
____, ____ & Tattersall, B. G. (2017a). × Dactylodenia lacerta R.M. Bateman & Tattersall: a morphologically cryptic hybrid orchid new to science from the Lizard Peninsula, Cornwall. New J. Bot. 7: 64 – 77.
____, Pridgeon, A. M. & Chase, M. W. (1997). Phylogenetics of subtribe Orchidinae (Orchidoideae, Orchidaceae) based on nuclear ITS sequences. 2. Infrageneric relationships and taxonomic revision to achieve monophyly of Orchis sensu stricto. Lindleyana 12: 113 – 141.
____ & Rudall, P. J. (2018). Clarified relationship between Dactylorhiza viridis and Dactylorhiza iberica renders obsolete the former genus Coeloglossum. Kew Bull. 73: 4 [17 pp.]
____, ____, Bidartondo, M. I., Cozzolino, S., Tranchida-Lombardo, V., Carine, M. A. & Moura, M. (2014). Speciation via floral heterochrony and presumed mycorrhizal host-switching of endemic butterfly orchids on the Azorean archipelago. Amer. J. Bot. 101: 979 – 1001.
____, ____ & Denholm, I. (2021b). In situ morphometric survey elucidates the evolutionary systematics of the orchid genus Gymnadenia in the British Isles. Syst. Biodivers. 19: 571 – 600.
____, ____, Murphy, A. R. M., Cowan, R. S., Devey, D. S. & Pérez-Escobar, O. A. (2021a). Even whole plastomes are not enough: phylogenomic and morphometric exploration at multiple demographic levels of the bee orchid clade Ophrys sect. Sphegodes. J. Exp. Bot. 72: 654 – 681.
____, Smith, R. J. & Fay, M. F. (2008). Morphometric and population-genetic analyses elucidate the origin, evolutionary significance and conservation implications of Orchis ×angusticruris (O. purpurea × O. simia), a hybrid orchid new to Britain. Bot. J. Linn. Soc. 157: 687 – 711.
____, Sramkó, G. & Paun, O. (2018b). Integrating restriction site-associated DNA sequencing (RAD-seq) with morphological cladistic analysis clarifies evolutionary relationships among major species groups of bee orchids. Ann. Bot. 121: 85 – 105.
Baum, A. & Baum, H. (2017). Platanthera muelleri — eine dritte Art in der Platanthera bifolia/chlorantha Gruppe in Mitteleuropa. J. Eur. Orch. 49: 133 – 152.
Bellusci, F., Pellegrino, G., Palermo, A. M. & Musacchio, A. (2008). Phylogenetic relationships in the orchid genus Serapias L. based on noncoding regions of the chloroplast genome. Molec. Phylogenet. Evol. 47: 986 – 991.
Bersweden, L. (2021). Species integrity and hybridization in anthropomorphic Orchis species. Doctoral thesis, Queen Mary University of London/Royal Botanic Gardens Kew.
____, Viruel, J., Schatz, B., Harland, J., Gargiulo, R., Cowan, R. S., Calevo, J., Juan, A., Clarkson, J. J., Leitch, A. R. & Fay, M. F. (2021). Microsatellites and petal morphology reveal new patterns of admixture in Orchis hybrid zones. Amer. J. Bot. 108: 1388 – 1404. DOI: https://doi.org/10.1002/ajb2.1710
Bidartondo, M. I., Burghardt, B., Gebauer, G., Bruns, T. D. & Read, D. J. (2004). Changing partners in the dark: isotopic and molecular evidence of ecomycorrhizal liaisons between forest orchids and trees. Proc. Roy. Soc. London, Ser. B, Biol. Sci. 271: 1799 – 1806.
Bilz, M., Kell, S. P., Maxted, N. & Lansdown, R. V. (2011). European Red List of Vascular Plants. Publications Office of the European Union, Luxembourg.
Bournérias, M., & D. Prat (eds) (2005). Les orchidées de France, Belgique et Luxembourg (2nd edn). Biotope, Mezé.
Bjerke, J. W. & Strann, K.-B. (2009). Orkideen tettbrudespore Gymnadenia densiflora i Nord-Norge. Blyttia 67: 126 – 133.
Braithwaite, M. E., Ellis, R. W. & Preston, C. D. (2006). Change in the British flora 1987 – 2004. BSBI, London.
Brandrud, M. K. (2019). The use of phylogenomic tools to investigate diploid and polyploid evolution in Dactylorhiza and other orchids. Doctoral thesis, University of Vienna.
____, Baar, J., Lorenzo, M. T., Athanasiadis, A., Bateman, R. M., Chase, M. W., Hedrén, M. & Paun, O. (2020). Phylogenomic relationships of diploids and the origins of allotetraploids in Dactylorhiza (Orchidaceae): RADseq data track reticulate evolution. Syst. Biol. 61: 91 – 109.
____, Paun, O., Lorenz, R., Baar, J. & Hedrén, M. (2019). Restriction-site associated DNA sequencing supports a sister group relationship of Nigritella and Gymnadenia. Molec. Phylogenet. Evol. 136: 21 – 28.
Breitkopf, H., Schlüter, P. M., Xu, S., Schiestl, F. P., Cozzolino, S. & Scopece, G. (2013). Pollinator shifts between Ophrys sphegodes populations: might adaptation to different pollinators drive population divergence? J. Evol. Biol. 26: 2197 – 2208.
Brys, R. & Jacquemyn, H. (2016). Severe outbreeding and inbreeding depression maintain mating system differentiation in Epipactis (Orchidaceae). J. Evol. Biol. 29: 352 – 359.
BSBI DDb. (2021). BSBI Distribution Database. https://database.bsbi.org/ [Accessed 18th August 2021].
Buttler, K.-P. (2011). Revision von Platanthera bifolia sensu lato. Jahrsber. Wetterauer Ges. Gesammte Naturk. Hanau 159 – 161: 93 – 108.
Campbell, V. V., Rowe, G., Beebee, T. J. C. & Hutchings, M. J. (2007). Genetic differentiation amongst fragrant orchids (Gymnadenia conopsea s.l.) in the British Isles. Bot. J. Linn. Soc. 155: 349 – 360.
Chalk, M. R. (2014). The sawfly orchid (Ophrys tenthredinifera) on the Dorset coast: a first for the British Isles? BSBI News #127: 32.
Chapurlat, E., Agren, J. & Sletvold, N. (2015). Spatial variation in pollinator-mediated selection on phenology, floral display and spur length in the orchid Gymnadenia conopsea. New Phytol. 208: 1264 – 1275.
____, Anderson, J., Agren, J., Friberg, M. & Sletvold, N. (2018). Diel pattern of floral scent emission matches the relative importance of diurnal and nocturnal pollinators in populations of Gymnadenia conopsea. Ann. Bot. 121: 711 – 721.
____, Le Ronce, I., Agren, J. & Sletvold, N. (2020). Divergent selection on flowering phenology but not only floral morphology between two closely related orchids. Ecol. Evol. 10: 5737 – 5747.
Charitonidou, M., Kougiomoutzis, K. & Halley, J. M. (2021). An orchid in retrograde: climate-driven range shift patterns of Ophrys helenae in Greece. Plants 10: 470.
____, ____, Karypidou, M. & Halley, J. (2022). 'Fly to a safer North': distributional shifts of the orchid Ophrys insectifera L. due to climate change. MDPI Biology 11 (4): 497.
Cheffings, C. M., Farrell, L., Dines, T. D., Jones, R. A., Leach, S. J., McKean, D. R., Pearman, D. A., Preston, C. D., Rumsey, F. J. & Taylor. I. (2005). The vascular plant Red Data List for Great Britain (Species Status 7). Joint Nature Conservation Committee, Peterborough.
Claessens, J., Gravendeel, B. & Kleynen, J. (2008). Cucullia umbratica L. als Bestaüber von Platanthera x hybrida Bruegg. in Süd-Limburg (Niederlande). J. Eur. Orch. 40: 73 – 84.
____ & Kleynen, J. (2011). The flower of the European orchid: form and function. Published by the authors, Voerendaal, Netherlands.
Clark, M. J. & Lewis, L. (2011). Discovery of Dactylorhiza praetermissa × Gymnadenia densiflora (Orchidaceae) at Kenfig National Nature Reserve, Glamorgan, Wales. New J. Bot. 1: 24 – 27.
____ & ____ (2018). Dactylorhiza incarnata ssp. lobelii, an Early Marsh-orchid new to Britain. BSBI News #138: 25 – 26.
Clark, N. (2021). Species of the month: Monkey Orchid, Orchis simia. Sussex Biodiversity Record Centre website (05/2021). https://sxbrc.org.uk/recording/speciesOfTheMonth.php?spMonth=43
Cole, S. R. (2014). History and status of the ghost orchid (Epipogium aphyllum, Orchidaceae) in England. New J. Bot. 4: 13 – 24.
____ & Waller, M. (2020). Britain's orchids. Princeton University Press, Princeton, NJ.
Conran, J., MacLunas, E. & MacLunas, K. (2011). Serapias lingua L. (tongue orchid) naturalised in the Adelaide Hills, South Australia: caveat cultivator. Orchadian 16: 556 – 561.
Curtis, T. G. F. & Thompson, R. (2009). The orchids of Ireland. National Museums Northern Ireland: Holywood, Co. Down.
Delforge, P. (2009). Orchis et monophylie. Naturalistes Belges 90: 15 – 35.
____ (2016). Orchidées d'Europe d'Afrique du Nord et du Proche-Orient. Delachaux & Niestlé, Paris.
____ & Gévaudan, A. (2002). Contribution taxonomique et nomenclaturale au groupe d’Epipactis leptochila. Naturalistes Belges 83: 19 – 35.
Devey, D. S., Bateman, R. M., Fay, M. F. & Hawkins, J. A. (2008). Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Ann. Bot. 101: 385 – 402.
____, ____, ____ & ____ (2009). Genetic structure and systematic relationships within the Ophrys fuciflora aggregate (Orchidinae: Orchidaceae): high diversity in Kent and a wind-induced discontinuity bisecting the Adriatic. Ann. Bot. 104: 483 – 495.
Devos, N., Raspé, O., Jacquemart, A.-L. & Tyteca, D. (2006). On the monophyly of Dactylorhiza Necker ex Nevski (Orchidaceae): is Coeloglossum (L.) Hartman a Dactylorhiza? Bot. J. Linn. Soc. 162: 261 – 269.
Dines, T. (2008). A vascular plant Red Data List for Wales (Rhestr o Blanhigion Fasgwlaidd Data Coch ar gyfer Cymru). Plantlife International, Salisbury.
Dueck, L. A., Aygoren, D. & Cameron, K. C. (2014). A molecular framework for understanding the phylogeny of Spiranthes (Orchidaceae), a cosmopolitan genus with a North American center of diversity. Amer. J. Bot. 101: 1551 – 1571.
Duffy, K. J., Kingston, N. E., Sayers, B., Roberts, D. L. & Stout, J. C. (2008). Inferring national and regional declines of rare orchid species with probabilistic models. Conserv. Biol. 23: 184 – 195.
Durka, W., Baum, A., Michalski, S. G. & Baum, H. (2017). Darwin's legacy in Platanthera: are there more than two species in the Platanthera bifolia/chlorantha group? Pl. Syst. Evol. 303: 419 – 431.
Eccarius, W. (2016). Die Orchideengattung Dactylorhiza. Published by the author, Bürgel.
Efimov, P. Machs, E., Liksakova, N. & Chetverikov, P. (2021). New records of Liparis kumokiri group (Orchidaceae) in Eurasia suggest its recent dispersal from East Asia. Russ. J. Biol. Inv. 12: 326 – 335.
Ennis, T. (2007). The occurrence of Dactylorhiza lapponica in Co. Antrim: a new Irish record. BSBI News #105: 13 – 15.
Eserman, L. A., Thomas, S. K., Coffey, E. E. D. & Leebens-Mack, J. H. (2021). Target sequence capture in orchids: developing a kit to sequence hundreds of single-copy loci. Applic. Pl. Sci. 9: e11416 [8 pp.].
Esposito, F., Jacquemyn, H., Waud, M. & Tyteca, D. (2016). Mycorrhizal fungal diversity and community composition in two closely related Platanthera (Orchidaceae) species. PLoS One 11: e0164108.
____, Merckx, T. & Tyteca, D. (2017). Noctuid moths as potential hybridization agents for Platanthera orchids. Lankesteriana 17: 383 – 393.
____, Vereecken, N. J., Gammella M., Rinaldi R., Laurent, P. & Tyteca, D. (2018). Characterization of sympatric Platanthera bifolia and Platanthera chlorantha (Orchidaceae) populations with intermediate plants. PeerJ 6: e4256.
Ettlinger, D. M. T. (1990). The 1976 Ophrys bertolonii in Dorset. BSBI News #54: 15 – 16.
Evans, A. & Jacquemyn, H. (2020). Impact of mating system on range size and niche breadth in Epipactis (Orchidaceae). Ann. Bot. 126: 1203 – 1214.
Fay, M. F. (2015). British and Irish orchids in a changing world. Curtis's Bot. Mag. 32: 3 – 23.
____ & Taylor, I. (2015). 801. Cypripedium calceolus. Curtis's Bot. Mag. 32: 24 – 32.
Foelsche, W. (2014). Die Gattung Nigritella im Lichte neuerer genetischer Untersuchungen mit besonderer Berücksichtigung von Nigritella miniata. Joannea Bot. 11: 89 – 160.
Forrest, A. D., Hollingsworth, M. L., Hollingsworth, P. M., Sydes, C. & Bateman, R. M. (2004). Population genetic structure in European populations of Spiranthes romanzoffiana set in the context of other genetic studies on orchids. Heredity 92: 218 – 227.
Garner, P. (2010). Haunted Herefordshire: the “Ghost” reappears in Britain after an absence of 23 years. BSBI News 113: 7.
Godfery, M. J. (1933). Monograph and iconograph of native British Orchidaceae. Cambridge University Press, Cambridge.
Good, R. (1936). On the distribution of lizard orchid (Himantoglossum hircinum Koch). New Phytol. 35: 142 – 170.
Greenwood, J. (2022). The Tiptree Tongue Orchids. J. Hardy Orch. Soc. 19(1): 17 – 24.
Groom, Q. J. (2013). Some poleward movement of British native vascular plants is occurring, but the fingerprint of climate change is not evident. PeerJ 1: e77.
Gupta, A. K., Schauvinhold, I., Pickersky, E. & Schiestl, F. P. (2014). Eugenol synthase genes in floral scent variation in Gymnadenia species. Funct. & Integr. Genom. 14: 779 – 788.
Hackel, J. & Sanmartin, I. (2021). Modelling the tempo and mode of lineage dispersal. Trends Ecol. Evol. 36: 1102 – 1112.
Haggar, J. & Malmgren, S. (2012). Much ado about almost nothing? J. Hardy Orch. Soc. 9 (3): 85 – 93.
Harrap, A. & Harrap, S. (2009). Orchids of Britain and Ireland. A. & C. Black, London.
Harris, S. A. & Abbott, R. J. (1997). Isozyme analysis of the reported origin of a new hybrid orchid species, Epipactis youngiana (Young's helleborine), in the British Isles. Heredity 79: 402 – 407.
Hedrén, M. (2009). Plastid DNA haplotype variation in Dactylorhiza incarnata (Orchidaceae): evidence for multiple independent colonization events into Scandinavia. Nord. J. Bot. 27: 69 – 80.
____, Fay, M. & Chase, M. W. (2001). Amplified fragment length polymorphisms (AFLP) reveal details of polyploid evolution in Dactylorhiza (Orchidaceae). Amer. J. Bot. 88: 1868 – 1880.
____, Lorenz, R., Teppner. H., Dolinar, B., Giotta, C., Griebl, N., Hansson, S., Heidtke, U., Klein, E., Perazza, G., Stahlberg, D. & Surina, B. (2018). Evolution and systematics of polyploid Nigritella (Orchidaceae). Nord. J. Bot. 36: njb-01539.
____, Nordström, S. & Bateman, R. M. (2011a). Plastid and nuclear DNA marker data support the recognition of four tetraploid marsh orchids (Dactylorhiza majalis s.l., Orchidaceae) in Britain and Ireland. Biol. J. Linn. Soc. 104: 107 – 128 (+ 4 electronic appendices).
____, ____ & Stahlberg, D. (2008). Polyploid evolution and plastid DNA variation in the Dactylorhiza incarnata/maculata complex (Orchidaceae) in Scandinavia. Molec. Ecol. 17: 5075 – 5091.
____, Paun, O. & Sayers, B. (2011b). The polymorphic early marsh orchids, Dactylorhiza incarnata s.l. (Orchidaceae), at Lough Gealain, Ireland. New J. Bot. 1: 16 – 23.
Henniges, M. C., Powell, R. F., Mian, S., Stace, C. A., Walker, K. J., Gornall, R. J., Christenhusz, M. J. M., Brown, M. R., Twyford, A. D., Hollingsworth, P. M., Jones, L., de Vere, N., Antonelli, A., Leitch, A. R. & Leitch, I. J. (2022). A taxonomic, genetic and ecological resource for the vascular plants of Britiain and Ireland. Nature: Sci. Data 9: 1.
Hertel, S. & Presser, H. (2019). Weitere Erkenntnisse zur Viefalt der Epipactis-Arten im südlichen Italien. Ber. Arbeitskreis. Heimische Orchid. 36: 5 – 61.
Hill, M. O. & Preston, C. D. (2015). Disappearance of boreal plants in southern Britain: habitat loss or climate change? Biol. J. Linn. Soc. 115: 598 – 610.
Hodkinson, T. R., Jones, M. B., Waldren, S. & Parnell, J. A. N. (eds) (2011). Climate change, ecology and systematics. Systematics Association Special Volume 78. Cambridge University Press, Cambridge.
Hollingsworth, P. M., Squirrell, J., Hollingsworth, M. L., Richards, A. J. & Bateman, R. M. (2006). Taxonomic complexity, conservation and recurrent origins of self-pollination in Epipactis (Orchidaceae). In: J. Bailey & R. G. Ellis (eds), Current taxonomic research on the British & European flora, pp. 27 – 44. Botanical Society of the British Isles, London.
____, and 51 co-authors (2009). A DNA barcode for land plants. Proc. Nat. Acad. Sci. USA 106: 12794 – 12797.
Hultén, E. (1950). Atlas över växternas utbredning i Norden. Generalstabens Litografiska Anstalts Förlag, Stockholm.
Hutchings, M. J., Robbert, K. M., Roberts, D. L. & Davy, A. J. (2018). Vulnerability of a specialized pollination mechanism to climate change revealed by a 356-year analysis. Bot. J. Linn. Soc. 186: 498 – 509.
IUCN. (2012). IUCN Red List Categories and Criteria. Version 3.1. Edn. 2. IUCN, Gland.
____ (Standards and Petitions Committee) (2019). Guidelines for using the IUCN Red List categories and criteria. Version 14.
____ (2021). The IUCN Red List of threatened species, v. 2021 – 2. https://www.iucnredlist.org
Jacquemyn, H., Brys, R. & Hutchings, M. J. (2011a). Biological flora of the British Isles 162: Orchis anthropophora (L.) All. (Aceras anthropophorum (L.) W.T. Aiton). J. Ecol. 99: 1551 – 1565.
____, ____, Lievens, B. & Wiegand, T. (2012). Spatial variation in below-ground seed germination and divergent mycorrhizal associations correlate with spatial segregation of three co-occurring orchid species. J. Ecol. 100: 1328 – 1337.
____, De Kort, H., Broeck, A. V. & Brys, R. (2018). Immigrant and extrinsic hybrid seed inviability contribute to reproductive isolation between forest and dune ecotypes of Epipactis helleborine (Orchidaceae). Oikos 127: 73 – 84.
____, ____, ____ & ____ (2020). Low genetic divergence and variation in coastal dune populations of the widespread terrestrial orchid Epipactis helleborine. Bot. J. Linn. Soc. 193: 419 – 430.
____, Merckx, V., Brys, R., Tyteca, D., Cammue, B. P. A., Honnay, O. & Lievens, B. (2011b). Analysis of network architecture reveals phylogenetic constraints of mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 192: 518 – 528.
____, Waud, M., Lievens, B. & Brys, R. (2016). Differences in mycorrhizal communities between Epipactis palustris, E. helleborine and its presumed sister species E. neerlandica. Ann. Bot. 118: 105 – 114.
Jannink, M. & Rich, T. (2010). Ghost Orchid rediscovered in Britain after 23 years. J. Hardy Orch. Soc. 7: 14 – 15.
Jin, W.-T., Jin, X.-H., Schuiteman, A., Li, D.-Z., Xiang, X.-G., Huang, W.-C., Li, J.-W. & Huang, L.-Q. (2014). Molecular systematics of subtribe Orchidinae and Asian taxa of Habenariinae (Orchideae, Orchidaceae) based on plastid matK, rbcL and nuclear ITS. Molec. Phylogenet. Evol. 77: 41 – 53.
____, Schuiteman, A., Chase, M. W., Li, J.-W., Chung, S.-W., Hsu, T.-C. & Jin, X.-H. (2017). Phylogenetics of subtribe Orchidinae s.l. (Orchidaceae; Orchidoideae) based on seven markers (plastid matK, psaB, rbcL, trnL-F, trnH-psba, and nuclear nrITS, Xdh): implications for generic delimitation. BMC Plant Biol. 17: 222 [14 pp.].
Kellenberger, R. T., Byers, K. J. R. P., Francisco, R. M. D. B., Staedler, Y. M., LaFountain, A. M., Schönenberger, J., Schiestl, F. P. & Schlüter, P. M. (2019). Emergence of a floral colour polymorphism by pollinator-mediated overdominance. Nature Comm. 10: 63 [11 pp.]
Keppel, G., Van Niel, K. P., Wardell-Johnson, G. W., Yates, C. J., Byrne, M., Mucina, L., Schut, A. G. T., Hopper, S. D. & Franklin, S. E. (2012). Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecol. Biogeogr. 21: 393 – 404.
Kitchener, G., Mills, D., Buckingham, S., Johnson, D. & Lemon, S. (2021). Serapias vomeracea Burm. f. (Briq.) (Long-lipped Tongue-orchid): first wild record for Britain and Ireland. BSBI News #146: 7 – 10.
Kohout, P., Tesitelova, T., Roy, M. Vohnik, M. & Jersakova, J. (2013). A diverse fungal community associated with Pseudorchis albida (Orchidaceae) roots. Fungal Ecol. 6: 50 – 64.
Kretzschmar, H., Eccarius, W. & Dietrich, H. (2007): The orchid genera Anacamptis, Orchis and Neotinea. EchinoMedia, Bürgel.
Kreutz, C. A. J. (2007). Epipactis dunensis (T. & T.A. Stephenson) Godfery subsp. tynensis Kreutz, Eine neue Epipactis-sippe aus Nordengland und Südschottland. J. Eur. Orch. 39: 123 – 134.
____ (2008). Update on British orchids. J. Hardy Orch. Soc. 5: 11 – 16.
____ (2019). Orchideeën van de Benelux. Kreutz Publishers, Sint Geertruid.
____, Dekker, H., Bons, G. & Löffler H. (2021). Veluwse wespenorchis (Epipactis veluwensis) een nieuwe Epipactis-soort in Nederland. Gorteria 43: 35 – 47.
____ & Lewis, L. (2015). Neotypification and distribution of Gymnadenia conopsea (L.) R. Brown var. friesica Schechter. J. Eur. Orch. 47: 239 – 249.
____, ____ & Giles, G. (2020). Epipactis neglecta var. collina, a new variety of Neglected Helleborine from England. Ber. Arbeitskreis. Heimische Orchid. 37 (2): 109 – 121.
Kühn, R., Pedersen, H. A. & Cribb, P. (2019). Field guide to the orchids of Europe and the Mediterranean. Royal Botanic Gardens, Kew.
Kropf, M. & Renner, S. (2008). Pollinator-mediated selfing in two deceptive orchids and a review of pollinium tracking studies addressing geitonogamy. Oecologia 155: 497 – 508.
Kull, T. & Hutchings, M. J. (2006). A comparative analysis of the decline of the distribution ranges of orchid species in Estonia and the United Kingdom. Biol. Conserv. 129: 31 – 39.
Lallemand, F., Figura, T., Damesin, C., Fresneau, C., Griveau, C., Fontaine, N., Zeller, B. & Selosse, M.-A. (2019). Mixotrophic orchids do not use photosynthates for perennial underground organs. New Phytol. 221: 12 – 17.
Lang, D. C. (2004). Britain’s orchids. Wild Guides, Old Basing, Hampshire.
____ & Spalton, L. M. (1998). Serapias lingua in South Devon (v.c.3). BSBI News #79: 20 – 21.
Leach, S. J. (2007). The vascular plant Red Data List for Great Britain: Year 1 amendments. BSBI News #104: 19 – 21.
____ (2010). The vascular plant Red Data List for Great Britain: Year 2 amendments. BSBI News 113: 43 – 44.
____ (2017). The vascular plant Red Data List for Great Britain: a summary of amendments in Years 10 and 11 (2015 – 16) of the annual amendments process. BSBI News #135: 59 – 62.
____ (2019). The vascular plant Red Data List for Great Britain: a summary of amendments in Years 12 and 13 (2017 – 18) of the annual amendments process. BSBI News #141: 3 – 7.
____ (2021). The vascular plant Red Data List for Great Britain: a summary of amendments in Years 14 and 15 (2019 – 20) of the annual amendments process. BSBI News #147: 27 – 35.
____ & Walker, K. J. (2011). The vascular plant Red Data List for Great Britain: a summary of Year 5 amendments, covering Years 3, 4 and 5 (2008 – 10) of the annual amendments process. BSBI News #116: 51 – 56.
____ & ____ (2013). The vascular plant Red Data List for Great Britain: a summary of amendments in Years 6 and 7 (2011 – 12) of the annual amendments process. BSBI News #123: 17 – 21.
____ & ____ (2015). The vascular plant Red Data List for Great Britain: a summary of amendments in Years 8 and 9 (2013 – 14) of the annual amendments process. BSBI News #128: 47 – 54.
Lewis, L. (2015). Reclassification of Gymnadenia conopsea var. friesica as a variety of Gymnadenia densiflora. J. Eur. Orch. 47: 293 – 302.
____, Clark, M. J. & Spencer, E. J. (2009). An update on the orchid flora at Kenfig National Nature Reserve. BSBI News #110: 7 – 11 + 1 plate.
____ & Spencer, E. J. (2005). An update on the orchid flora at Kenfig National Nature Reserve. Watsonia 25: 290 – 295.
Li, J.-H., Liu, Z.-J., Salazar, G. A., Bernhardt, P., Perner, H., Tomohisa, Y., Jin, X.-H., Chung, S.-W. & Luo, Y.-B. (2011). Molecular phylogeny of Cypripedium (Orchidaceae: Cypripedioideae) inferred from multiple nuclear and chloroplast regions. Molec. Phylogenet. Evol. 61: 308 – 320.
Lobas, Z., Khapugin, A., Zolubak, E. & Jakubska-Busse, A. (2021). The Epipactis helleborine group (Orchidaceae): an overview of recent taxonomic changes, with an updated list of currently accepted taxa. Plants 10, 1839 [13 pp.]
Lorenz, R., Akhalkatsi, M., Cortis, P., Galesi, R., Giotta, C., Madl, J., Obrist, E., Picitto, M., Romano, V. A., Romolini, R. & Soca, R. (2015). Morphometrische Untersuchungen zur Variabilität und Gliederung der Gattung Platanthera in Italien. J. Eur. Orch. 47: 123 – 238.
Lousley, J. E. (1976). Flora of Surrey. David & Charles, Newton Abbott, Devon.
Lussu, M., De Agostini, A., Cogoni, A., Marignani, M. & Cortis, P. (2019). Does size really matter? A comparative study on floral traits in orchids with two different pollination strategies. Pl. Biol. 21: 961 – 966.
Madge, S. (1994). The status of Serapias parviflora Parl. in Britain. Botanical Cornwall 6: 51 – 52.
Meekers, T., Hutchings, M. J., Honnay, O. & Jacquemyn, H. (2012). Biological flora of the British Isles: Gymnadenia conopsea s.l. J. Ecol. 100: 1269 – 1288.
Molnár, A. V. (ed.) (2011). Magyarország Orchideáinak Atlasza. Kossuth Kiadó, Budapest.
Mötlep, M., Ilves, A., Tali, K., Sild, E. & Kull, T. (2021). Artificial crossing and pollen tracking reveal new evidence of hybridisation between sympatric Platanthera species. Pl. Syst. Evol. 307: 25 [14 pp.]
Olson, P. D., Hughes, J. & Cotton, J. A. (eds) (2016). Next generation systematics. Systematics Association Special Volume 85. Cambridge University Press, Cambridge.
Pankhurst, R. G. & Matthews, G. A. (1977). Ophrys bertolonii Moretti in Britain. Watsonia 11: 430.
Parker, S. (2016). Wild orchids of Wales. First Nature, Ceredigion.
Parsons, E. C. M. (2016). Why IUCN should replace “Data Deficient” conservation status with a precautionary “Assume Threatened” status: a cetacean case study. Front. Marine Sci. 3: 193 [3 pp.]
Paun, O., Bateman, R. M., Fay, M. F., Hedrén, M., Civeyrel, L. & Chase, M. W. (2010). Stable epigenetic effects impact evolution and adaptation in allopolyploid orchids. Molec. Biol. Evol. 27: 2465 – 2473.
Pedersen, H. A. (1998). Species concept and guidelines for infraspecific taxonomic ranking in Dactylorhiza (Orchidaceae). Nord. J. Bot. 18: 289 – 310.
____ (2001). Late-flowering dune populations of Dactylorhiza incarnata (Orchidaceae): variation patterns and taxonomic inferences. Nord. J. Bot. 21 (2): 177 – 186.
____ (2007). Taxonomic revision of the Dactylorhiza majalis ssp. purpurella complex (Orchidaceae): a morphometric approach. J. Eur. Orch. 39: 341 – 366.
Pérez-Escobar, O. A., Dodsworth, S., Bogarin, D., Bellot, S., Balbuena, J. A., Schley, R., Kikuchi, I., Morris, S. K., Epitawalage, N., Cowan, R., Maurin, O., Zuntini, A., Arias, T., Serna, A., Gravendeel, B., Fernanda-Torres, M., Nargar, K., Chomicki, G., Chase, M. W., Leitch, I. J., Forest, F. & Baker, W. J. (2021). Hundreds of nuclear and plastic loci yield insights into orchid relationships. Amer. J. Bot. 108: 1166 – 1180.
Perring, F. H. & Walters, S. M. (1962). Atlas of the British flora (1st edn). Nelson, London.
____ & ____ (1982). New atlas of the British flora (3rd edn). Oxford University Press, Oxford.
Pettorelli, N., Smith, J., Pecl, G. T., Hill, J. K. & Norris, K. (2019). Anticipating arrival: tackling the international challenges associated with the redistribution of biodiversity driven by climate change. J. Appl. Ecol. 56: 2298 – 2304.
Pillon, Y., Fay, M. F., Hedrén, M., Bateman, R. M., Devey, D., Shipunov, A., van der Bank, M. & Chase, M. W. (2007). Insights into the evolution and biogeography of Western European species complexes in Dactylorhiza (Orchidaceae). Taxon 56: 1185 − 1208.
Pineiro-Fernández, L., Byers, K. J. R. P., Cai, J., Sedeek, K. E. M., Kellenberger, R. T., Russo, A., Qi, W., Aquino-Fournier, C. & Schlüter, P. M. (2019). Phylogenomic analysis of the floral transcriptomes of sexually deceptive and rewarding European orchids, Ophrys and Gymnadenia. Front. Pl. Sci., 01553.
Preston, C. D., Pearman, D. A. & Dines, T. D. (2002). New atlas of the British and Irish flora. Oxford University Press, Oxford.
Rankou, H. (2011). Gymnadenia borealis. The IUCN Red List of threatened species 2011: e.T175941A7149754
Raskoti, B. B., Schuiteman, A., Jin, W.-T. & Jin, X.-H. (2017). A taxonomic revision of Herminium L. (Orchidoideae, Orchidaceae). PhytoKeys 79: 1 – 74.
Rich, T. C. G. (2003). Flowering plants. In: D. L. Hawksworth (ed.), The changing wildlife of Great Britain and Ireland, pp. 23 – 49. Taylor & Francis, London.
Richards, A. J. (1994). Epipactis leptochila s.l. In: A. Stewart, D. A. Pearman & C. D. Preston (eds), Scarce plants in Britain, p. 152. Joint Nature Conservation Committee, Peterborough.
____ (1998). Epipactis. In: T. C. G. Rich & A. C. Jermy (eds), Plant Crib 1998, pp. 378 – 379. Botanical Society of the British Isles, London.
____ & Porter, A. F. (1982). On the identity of a Northumberland Epipactis. Watsonia 14: 121 – 128.
____ & Squirrell, J. (2009). Epipactis leptochila complex in Britain. BSBI News, #112: 8 – 9.
Rose, F. (1988). Gymnadenia. In: T. G. C. Rich & A. C. Jermy (eds), The plant crib (1st edn), pp. 118 – 119. Botanical Society of the British Isles, London.
Roy, M., Gonneau, C., Rocheteau, A., Berveiller, D., Thomas, J. C., Damesin, C. & Selosse, M. A. (2013). Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol. Monogr. 83: 95 – 117.
Salazar, G. A., Cabrera, L. I., Madrinan, S. & Chase, M. W. (2009). Phylogenetic relationships of Cranichidinae and Prescotiinae (Orchidaceae, Cranichideae) from plastid and nuclear DNA sequences. Ann. Bot. 104: 403 – 416.
____ and 20 coauthors (2018). Phylogenetic systematics of subtribe Spiranthinae (Orchidaceae: Orchidoideae:Cranichideae) based on nuclear and plastid DNA sequences of a nearly complete generic sample. Bot. J. Linn. Soc. 186: 273 – 303.
Santos, G. & Sayers, B. (2021). Epipactis dunensis (Orchidaceae): a confirmed new addition for the Irish flora. ?Privately published, ?Dublin [6 pp.]
Sardaro, M. L. S., Atallah, M., Picarella, M. E. Aracri, B. & Pagnotta, M. A. (2012). Genetic diversity, population structure and phylogenetic inference among Italian Orchids of the Serapias genus assessed by AFLP molecular markers. Pl. Syst. Evol. 298: 1701 – 1710.
Schiebold, J. M. I., Bidartondo, M. I., Karasch, P., Gravendeel, B. & Gebauer, G. (2017). You are what you get from your fungi: nitrogen stable isotope patterns in Epipactis species. Ann. Bot. 119: 1085 – 1095.
Scopece, G., Musacchio, A., Widmer, A. & Cozzolino, S. (2007). Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution 61: 2623 – 2642.
Sedeek, K. E. M., Whittle, E., Guthörl, D., Grossniklaus, U., Shanklin, J. & Schluter, P. M. (2016). Amino acid change in an orchid desaturase enables mimicry of the pollinator's sex pheromone. Curr. Biol. 26: 1505 – 1511.
Sell, P. D. & Murrell, G. (1996). Flora of Great Britain and Ireland, 5: Butomaceae–Orchidaceae. Cambridge University Press, Cambridge.
Serna-Sánchez, M., Pérez-Escobar, O. A., Bogarin, D., Torres-Jimenez, M. F., Alvarez-Yela, A. C., Arcila-Galves, J. E., Hall, C. F., de Barros, F., Pinheiro, F., Dodsworth, S., Chase, M. W., Antonelli, A. & Arias, T. (2021). Plastid phylogenetics resolves ambiguous relationships within the orchid family and provides a solid timeframe for biogeography and macroevolution. Nature Sci. Rep. 11: 6858 [11 pp.]
Squirrell, J., Hollingsworth, P. M., Bateman, R. M., Dickson, J. H., Light, M. H. S., McConaill, M. & Tebbitt, M. C. (2001). Partitioning and diversity of nuclear and organelle markers in native and introduced populations of Epipactis helleborine (Orchidaceae). Amer. J. Bot. 88: 1409 – 1418.
____, ____, ____, Tebbitt, M. C. & Hollingsworth, M. L. (2002). Taxonomic complexity and breeding system transitions: conservation genetics of the Epipactis leptochila complex (Orchidaceae). Molec. Ecol. 11: 1957 – 1964.
Sramkó, G., Molnár, A. V., Hawkins, J. A. & Bateman, R. M. (2014). Molecular phylogenetics and evolution of the Eurasiatic orchid genus Himantoglossum s.l. Ann. Bot. 114: 1609 – 1626.
____, Paun, O., Brandrud, M. K., Laczko, L., Molnár, A. V. & Bateman, R. M. (2019). Iterative allogamy–autogamy transitions drive actual and incipient speciation during the ongoing evolutionary radiation within the orchid genus Epipactis (Orchidaceae). Ann. Bot. 124: 481 – 497.
Stace, C. A. (1991). New flora of the British Isles (1st edn). Cambridge University Press, Cambridge.
____ (1997). New flora of the British Isles (2nd edn). Cambridge University Press, Cambridge.
____ (2010). New flora of the British Isles (3rd edn). Cambridge University Press, Cambridge.
____ (2019). New flora of the British Isles (4th edn). Cambridge University Press, Cambridge.
____, Preston, C. D. & Pearman, D. A. (2016). Hybrid flora of the British Isles. BSBI, Bristol.
Stahlberg, D. & Hedrén, M. (2010). Evolutionary history of the Dactylorhiza maculata polyploid complex (Orchidaceae). Biol. J. Linn. Soc. 101: 503 – 525.
Stark, C., Michalski, S. G., Babik, W., Winterfeld, G. & Durka, W. (2011). Strong genetic differentiation between Gymnadenia conopsea and G. densiflora despite morphological similarity. Pl. Syst. Evol. 293: 213 – 226.
Stroh, P. (2021). County roundups 2021: England. BSBI News #148: 65 – 67.
____, Leach, S. J., August, T. A., Walker, K. J., Pearman, D. A., Rumsey, F. J., Harrower, C. A., Fay, M. F., Martin, J. P., Pankhurst, T., Preston, C. D. & Taylor, I. (2014). A vascular plant Red List for England. Botanical Society of Britain and Ireland, Bristol.
____, Walker, K. J., Humphrey, T. & Pescott, O. L. (eds). (2022). Plant Atlas 2000 – 2019: Mapping the Distribution of the British and Irish Flora. Botanical Society of Britain and Ireland/Princeton University Press, New Jersey.
Summerhayes, V. S. (1951). Wild orchids of Britain (1st edn). Collins, London.
Swainbank, T. (2017). Is the hybrid between Britain's two butterfly orchids, Platanthera ×hybrida, under-recorded? BSBI News #135: 51 – 58.
____ & Boseley, K. (2021). Distinguishing between Epipactis leptochila (Narrow-lipped Helleborine), Epipactis helleborine (Broad-leaved Helleborine) and their putative hybrid, Epipactis x stephensonii (Orchidaceae) at the Warburg Reserve, Oxfordshire. British & Irish Botany 3: 196 – 215.
Tang, Y., Yukawa, T., Bateman, R. M., Jiang, H. & Peng, H. (2015). Phylogeny and classification of the East Asian Amitostigma alliance (Orchidaceae: Orchideae) based on six DNA markers. BMC Evol. Biol. 15: e96 [32 pp.] [doi: https://doi.org/10.1186/s12862-015-0376-3]
Taylor, A., Weigelt, P., König, C., Zotz, G. & Kreft, H. (2019). Island disharmony revisited using orchids as a model group. New Phytol. 223: 597 – 603.
Taylor, L. & Roberts, D. L. (2011). Biological flora of the British Isles. No. 261. Epipogium aphyllum Sw. J. Ecol. 99: 878 – 890.
Temple, W. (2011). Comment on Hartslock hybrids. J. Hardy Orch. Soc. 8(1): 6.
Teppner, H. (2004). A review of new results in Nigritella (Orchidaceae). Sprawozdania z posiedzen komisji naukowych 46: 111 – 116.
Thompson, J. B., Dodd, H. O., Wills, M. A. & Priest, N. K. (2021). Speciation across the Earth driven by global cooling in orchidoid orchids. BioRxiv. https://doi.org/10.1101/2021.02.06.430029
Trávníček, P., J. Jersáková, B. Kubátová, J. Krejčíková, R. M. Bateman & 22 co-authors (2012). Minority cytotypes in European populations of the Gymnadenia conopsea complex (Orchidaceae) greatly increase intraspecific and intrapopulation diversity. Ann. Bot. 110: 977 – 986.
____, Chumova, Z., Záveská, E., Hanzlíčková, J., Kupková, L., Kučera, J., Gbúrová Štubnová, E., Rejlová, L., Mandakova, T. & Ponert, J. (2021). Integrative study of genotypic and phenotypic diversity in the Eurasian orchid genus Neotinea. Front. Plant Sci. 12: 734240.
Trudgill, D. L. (2017). Orchids — conservation versus wildness. BSBI News #136: 20 – 22.
____ (2019). Establishment of Pyramidal Orchid and Broad-leaved Helleborine in our meadow in Scotland. J. Hardy Orch. Soc. 16 (3): 91 – 95.
____ (2022). Orchids in Scotland: changes in their occurrence since 1950. Brit. Irish Bot. 4: 34 – 41.
Trunschke, J., Sletvold, N. & Agren, J. (2020). Manipulation of trait expression and pollination regime reveals the adaptive significance of spur length. Evolution 74: 597 – 609.
Tyteca, D., Ceinos, M., Gathoye, J.-L., Brys, R. & Jacquemyn, H. (2012). On the morphological, biological and genetic heterogeneity of the genus Orchis (Orchidaceae, Orchidinae). Phytotaxa 75: 19 – 32.
____ & Esposito, F. (2018). Recent proposals in Platanthera (Orchidaceae) systematics in Western Europe, with focus on intermediate looking plants. J. Eur. Orch. 50: 393 – 408.
____ & Klein, E. (2008). Genes, morphology and biology – the systematics of Orchidinae revisited. J. Eur. Orch. 40: 501 – 544.
Vereecken, N. J., Dafni, A. & Cozzolino, S. (2010). Pollination syndromes in Mediterranean orchids — implications for speciation, taxonomy and conservation. Bot. Rev. 76: 220 – 240.
Vermeulen, P. (1949). Varieties and forms of Dutch orchids. Ned. Kruidk. Arch. 56: 204 – 242.
Walker, K. J. (2008). The Threatened Plants Project (TPP) — a national pilot for 2008. BSBI News #107: 69 – 70.
____, Stroh, P. A. & Ellis, R. W. (2017). Threatened plants in Britain and Ireland. Botanical Society of Britain and Ireland, Bristol.
Waller, M. & Cole, S. (2017). Serapias lingua discovered in Essex. BSBI News #136: 11 – 12.
Walters, S. M. (1984). The relation between the British and the European floras. New Phytol. 98: 3 – 13.
Wigginton, M. J. (1999). British Red Data Books. 1. Vascular Plants (edn 3). Joint Nature Conservation Committee, Peterborough.
Wilmott, A. J. (1936). New British marsh orchids. Proc. Linn. Soc. London 148: 126 – 130.
Wood, J. & Ramsay, M. (2004). 482. Anacamptis laxiflora (Orchidaceae). Curtis's Bot. Mag. 21: 26 – 33.
Wróblewska, A., Szczepaniak, L., Bajguz, A., Jedrzejczyk, I., Talalaj, I., Ostrowiecka, B., Brzosko, E., Jermakowicz, E. & Mirski, P. (2019). Deceptive strategy in Dactylorhiza orchids: multidirectional evolution of floral chemistry. Ann. Bot. 123: 1005 – 1016.
Wyse Jackson, M., FitzPatrick, Ú., Cole, E., Jebb, M., McFerran, D., Sheehy Skeffington, M. & Wright, M. (2016). Ireland Red List No. 10: Vascular Plants. National Parks and Wildlife Service, Department of Arts, Heritage, Regional, Rural and Gaeltacht Affairs, Dublin.
Young, D. P. (1962). Studies in the British Epipactis, 5. Epipactis leptochila; with some notes on E. dunensis and E. muelleri. Watsonia 5: 127 – 135.
Zhou, T. & Jin, X.-H. (2018). Molecular systematics and the evolution of mycoheterotrophy of tribe Neottieae (Orchidaceae, Epidendroideae). PhytoKeys 94: 39 – 49.
Acknowledgements
I thank Pete Stroh for much helpful advice when preparing the captions for the orchid distribution maps in Atlas 2020, Ian Denholm, Sean Cole and Jess Stone for allowing me to include their images in Fig. 6, and all of the research collaborators whose collective competence and generosity has contributed to this work. I am grateful to Paula Rudall, Sean Cole and Oscar Pérez-Escobar for critically reading the manuscript. This paper is dedicated to the late Victor Summerhayes, whose 1968 second edition of Wild orchids of Britain played a crucial role in developing my life-long obsession with our native orchids.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bateman, R.M. Systematics and conservation of British and Irish orchids: a "state of the union" assessment to accompany Atlas 2020. Kew Bull 77, 355–402 (2022). https://doi.org/10.1007/s12225-022-10016-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10016-5