Abstract
The genus Codium is one of the most important genera of marine green macroalgae. Its distribution is widespread worldwide and it has a high degree of diversity in species and characteristics. This genus plays an important ecological role in marine ecosystems as it is a primary producer. However, some species in the genus Codium are invasive species and may disturb the functioning of the ecosystem. Economically, Codium has promising potential as a source of diverse nutritional and pharmacological compounds. Codium is edible, has a high nutrient value, and is rich in bioactive compounds. Hence, some species of Codium have been consumed as food and used as herbal medicines in some Asian countries. In recent decades, studies of the bioactivity and pharmacological properties of the genus Codium have attracted the attention of scientists. This review aims to identify gaps in studies analyzing Codium that have been conducted in the past three decades by assessing published research articles on its bioactivity and pharmacological properties. Compounds obtained from Codium have demonstrated significant biological activities, such as immunostimulatory, anticoagulant, anticancer, anti-inflammatory, antioxidant, antiviral, antibacterial, antifungal, antitumor, anti-angiogenic, osteoprotective, and anti-obesity activities. This review provides information that can be used as a future guideline for sustainably utilizing the genus Codium.
Similar content being viewed by others
Introduction
Codium (Bryopsidales) is a diverse genus of marine green macroalgae belonging to the Codiaceae family (Verbruggen et al. 2007). Codium has attracted global attention because of its high biodiversity, ecological features as an invasive species, and high potential for producing bioactive compounds. The genus Codium comprises approximately 166 species that are distributed in marine environments throughout the world and have been cultivated in some countries (Verbruggen et al. 2007; Hwang et al. 2008; Kang et al. 2008; Guiry and Guiry 2022; Hwang and Park 2020). Recently, molecular identification of Codium species has been used to avoid their misidentification, owing to their high morphological plasticity (Provan et al. 2008; de Oliveira-Carvalho et al. 2012; Verbruggen 2014; Verbruggen and Costa 2015; Muha et al. 2019). Some species of Codium that have been identified earlier are C. coactum Okamura, C. contractum Kjellman, C. fragile (Suringar) Hariot, and C. minus (Schmidt) P.C. Silva (Woo and Sook 2015). Some new Codium species that have been identified in recent years include C. bernabei (González et al. 2012), C. pernambucensis (de Oliveira-Carvalho et al. 2012), C. recurvatum (Verbruggen et al. 2012), and C. lucasii (An et al. 2015). Codium fragile, one of the most popular and edible green algae species, is also one of the most invasive species originating from the Northwest Pacific (Japan) (Provan et al. 2008). This species then spread to the Northeast Pacific, the North Atlantic, Australia, and New Zealand (Dromgoole 1975; Schmidt and Scheibling 2005; Muha et al. 2019). Species of Codium play an important role in marine ecosystems. Some of them are invasive species that can disturb marine ecosystems but can also have a balance impact if they coexist with other Codium species.
Codium has become one of the main macroalgae consumed in some Asian countries, such as Japan, China, and Korea. Codium has high nutritional properties, including its composition of carbohydrates, proteins, lipids, vitamins, and minerals (Tabarsa et al. 2013; Jung and Park 2020; Monmai et al. 2020), as well as bioactive compounds, such as siphonaxanthin (Akimoto et al. 2007; Ganesan et al. 2010), canthaxanthin (Ahn et al. 2021), oleamide (Moon et al. 2018b), and sulfated polysaccharides (Wang et al. 2021). Recently, sulfated polysaccharides from Codium species such as C. pugniforme, C. yezoense, C. latum, and C. vermilara were identified as sulfated glucan, sulfated galactan, sulfated arabinan, and sulfated mannan (Bilan et al. 2006; Fernández et al. 2012, 2014; Li et al. 2015). Bioactive compounds and polysaccharides present in Codium possess interesting pharmacological effects, including immunostimulatory (Yang et al. 2019, 2021), anti-inflammatory (Yoon et al. 2011; Moon et al. 2018b), anticancer (Hye et al. 2018), anticoagulant (Choi et al. 2013), antioxidant (Wang et al. 2020), anti-obesity (Kolsi et al. 2017a, b), osteoprotective (Surget et al. 2017), and antiviral (Yim et al. 2021) activities. However, the ecology, nutrient value, bioactive compound composition, and bioactivity of Codium have not yet been comprehensively reviewed to determine the gap in studies analyzing Codium, which can be used as a direction for future studies and management of the genus Codium.
Distribution of genus Codium
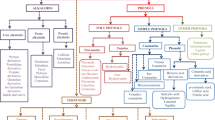
The genus Codium is found worldwide (Fig. 1). The green alga Codium is believed to have certain invasive properties because of its ability to thrive in temperate waters. Codium tomentosum (Stackhouse, 1797) is native to the northeast Atlantic coast and inhabits in rock pools and lower seashores throughout the year (Rey et al. 2020). Codium decorticatum (Woodward) M.A. Howe is a species found in tropical and subtropical climates worldwide. There are 105 subspecies of C. decorticatum along the Atlantic coast of South America, ranging latitudinally from 3°S to 42°S (Fernández et al. 2015). This species grows on firm substrates in subtidal habitats. Codium bursa (Olivi) C. Agardh is typically found in temperate and subtropical climates. It can grow in diameters ranging from a few millimeters to 40 cm, and it grows in a hollow spherical form (Jerkovi et al. 2019). Some species of the genus Codium are invasive. Among the species within Codium, C. fragile is the most invasive seaweed in the world and is believed to be native to Japan, from which it accidentally spread to other parts of the world (Provan et al. 2008). Native to East Asia, it has invaded many parts of the world and now has a nearly global distribution (Hubbard and Garbary 2002; Provan et al. 2005; Schmidt and Scheibling 2005).
The habitat of Codium is rocky substrate in the intertidal zone. Sheltered rocky habitats are critical for Codium as these habitats allow for algae to grow and reproduce (Bulleri et al. 2006; Woo and Sook 2015). In addition to the habitat, other ecological factors also affect the characteristics of Codium. Seasonal patterns affect the morphology and chloroplast physiology (Benson et al. 1983), growth (Hanisak 1979), reproductive characteristics (Churchill and Moeller 1972; Prince and Trowbridge 2004), and the nutritional value of Codium (Malea et al. 2015). Furthermore, water movement and substratum type may contribute to the vegetative recruitment ability (Scheibling and Gagnon 2006) and the formation and growth of spongy and filamentous thalli (Nanba et al. 2005). In new habitats, they can have ecological and economic impacts; for example, they may compete with native kelps or fucoids (Scheibling and Gagnon 2006; Drouin et al. 2011; Armitage and Sjøtun 2016), influence the seaweed-associated fauna composition (Schmidt and Scheibling 2006; Drouin et al. 2011; Armitage and Sjøtun 2016), negatively affect commercial bivalve beds, change the sediment from sand to pebbles and cobbles (Ben-Avraham 1971), and impact ecosystem services (Vilà et al. 2010). In addition to nutrient over-enrichment, the invasion by non-native species has been detrimental to biodiversity and ecosystem functioning in many coastal ecosystems (Thomsen et al. 2006).
Biochemical properties
Marine macroalgae are rich sources of new bioactive compounds and functional foods with potentially beneficial health effects (Kim et al. 2020). They have been reported to have nutritional value due to their vitamin, protein, and mineral content (Ortiz et al. 2009; Holdt and Kraan 2011; El-Said and El-Sikaily 2013; Lafarga et al. 2020). Marine macroalgae contain protein, carbohydrate, and low-fat, hence, they can contribute a few calories to the diet (Rupérez 2002). The variations in the nutritional composition of algae may be influenced by complex endogenous growth-related, morphological, and reproductive changes, as well as exogenous factors including temperature, light intensity, day length, and concentration of nutrients (Stirk et al. 2007; Rey et al. 2020; Marques et al. 2021).
Sulfated polysaccharides
Green macroalgae contain different typical carbohydrates, including cellulose, xylan, and sulfated polysaccharides. There is a lack study on the structures of sulfated polysaccharides from marine green macroalgae compare to those from marine red or brown macroalgae (Farias et al. 2008). The structural heterogeneity of sulfated polysaccharides within Codium species are different for each species (Fig. 2). The unusual complex pyruvylated and sulfated galactans in C. yezoense consist of linear backbone units of 3-linked β-D-galactopyranosyl components divided by oligosaccharides connected by links at C6 (Bilan et al. 2007). The sulfated galactan of C. isthmocladum primarily consists of 4-sulfated 3-linked β-D-galactopyranosyl units (Farias et al. 2008). A family of sulfated polysaccharides, including sulfated arabinans, sulfated galactans and sulfated arabinogalactans as the main components, was found in the room-temperature water extracts of C. fragile and C. vermilara (Ciancia et al. 2007; Estevez et al. 2009). Moreover, the sulfated polysaccharide in C. latum, C. pugniforme (syn. C. spongiosum),and C. vermilara were described as sulfated arabinan, sulfated glucan, and sulfated mannan, respectively (Bilan et al. 2006; Fernández et al. 2012, 2014). Sulfated galactans from C. fragile differs from the C. cylindricum. Regarding to its galactose content, C. fragile also contains arabinose residues or known as sulfated arabinogalactan (Love and Percival 1964), and C. cylindricum (syn. C. divaricatum) contains glucose residues, probably forming sulfated glucogalactan (Matsubara et al. 2001). The analysis of sulfated galactans from various Codium species has revealed that 3-linked β-D-galactopyranosyl has comparable backbones. The structures of the sulfated polysaccharides directly affect their biological activities in regards to their main structure, molecular weight, degree of sulfation, monosaccharide composition, and glycosidic linkages (Sabry et al. 2019).
Lipids
Lipid is a component in macroalgae that has attracted attention due to its fatty acid fraction. Polyunsaturated fatty acids (PUFAs) are essential lipids for human metabolism. However, human can not synthesize them and must obtain them through their daily intake. The major PUFAs detected in macroalgae were C18 and C20 PUFAs, namely linoleic, arachidonic and eicosapentaenoic acids (Pereira et al. 2012). In Chlorophyta, the PUFAs content ranges from 17–61% with α-linolenic acid as the most abundant fatty acid (Allan et al. 2010; Goecke et al. 2010; Pereira et al. 2012; Schmid et al. 2018). Meanwhile, in Codium lipids are mostly in the form of LFA and SFA with an unusual structure of fatty acids. Long-chain fatty acids are present in Codium species, with palmitic acid being the most common saturated fatty acid (SFA) and oleic acid being the most common monounsaturated fatty acid (MUFA) (Shameel 1990). The content of fatty acids in Codium varies depending on various factors, such as species, growth age, nutrient, season, temperature, salinity, location, and depth (Xu et al. 1998; Dembitsky and Hanus 2003). Codium species contain an unusual structure of several branched fatty acids (Aliya and Shameel 1993; Dembitsky and Hanus 2003). Codium tomentosum contains α-linolenic acid, palmitic, palmitoleic, oleic, hexadecatrienoic, eicosatrienoic and eicosapentaenoic acids (da Costa et al. 2015). Meanwhile, α-linolenic, palmitic acid, oleic, linoleic, and hexadecatrienoic acids were detected in C. fragile, C. tomentosum, C. geppi and Codium sp. (Khotimchenko 2003). Moreover, Ortiz et al. (2009) observed that the most abundant fatty acids in C. fragile was palmitic acid. Aliya and Shameel (1993) investigated 43 different fatty acids which identified as methyl esters fatty acid in C. decorticatum, C. flabellatum, and C. iyengarii. Dembitsky and Hanus (2003) investigated fatty acid variability of C. dwarkense and C. taylorii and identified 40 volatile compounds of monoenoic acid and polyenoic acid. Furthermore, these three Codium species contained eight sterols including ergosterol, ostreasterol, clerosterol, decortinone, decortinol, isodecortinol, cholesteryl acetate and cholesteryl galactoside (Aliya and Shameel 1993).
Proteins
The protein content of marine macroalgae is also variable and the highest content is generally found in marine green and red algae, compared to brown algae (Holdt and Kraan 2011). Codium tomentosum is known to contain 11.00–18.8% dw of total protein (Celikler et al. 2009; Rodrigues et al. 2015). A similar protein content was also found in C. galeatum (12% dw) and C. fragile (10.8% dw) (Ortiz et al. 2009; Skrzypczyk et al. 2018). Bioactive compounds derived from proteins such as lectins can be obtained from Codium species. Carneiro et al. (2020) isolated lectins from C. isthmocladum and found two novel lectins, CiL-1 and CiL-2, with unique sequences not found in other lectins.
Minerals and vitamins
Minerals and vitamins are present in macroalgae at high levels and have received considerable attention because the macrominerals and trace elements content in macroalgae are comparable to land-plants and can be used to fulfill human daily needs intake (Rupérez 2002). Macroalgae can selectively absorb minerals from the surrounding seawater and accumulate them in their cells (Cabrita et al. 2016). As for the major minerals, most macroalgae show abundant contents of sodium (Na), magnesium (Mg), potassium (K), and calcium (Ca). As expected, high Na content was found in C. fragile and C. tomentosum (92.3 and 11.79 mg g−1 dw), (Moreda-Pineiro et al. 2012; El-Said and El-Sikaily 2013), while C. iyengarii contains high K (231.7 mg g−1 dw) (Rizvi and Shameel 2004). However, the availability of minerals content in marine macroalgae is influenced by intrinsic and extrinsic factors. The intrinsic factors are including specific forms of hydroxyl, carboxyl, amino, and sulfhydryl esters functional groups from their polysaccharides, lipid, and proteins, and extrinsic factors are including pH, temperature, salinity, and other external factors in the growth medium (Circuncis et al. 2018).
Trace elements are classified into two subclasses: (a) cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn), which are required for biochemical processes but may be toxic at high concentrations, and (b) arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), and mercury (Hg), which are not required for biochemical processes but are the most important contaminants in aquatic environments. Among the trace minerals, strontium (Sr), barium (Ba), and Fe were found in high concentrations in C. fragile (Malea et al. 2015; Seo et al. 2019). A high content of Fe is present in C. reediae (91.0–196.0 μg g−1 dw) (Mcdermid and Stuercke 2003). For heavy metals, As exhibited the highest content (4.25 μg g−1 dw), while Cd exhibited the lowest content (0.05 μg g−1 dw) in C. fragile (Malea et al. 2015). Heavy metal contamination is a factor that is used to assess the safety of edible macroalgae (Zheng et al. 2013).
Macroalgae contain more vitamins A, B-12, and C, β-carotene, pantothenate, folate, riboflavin, and niacin than fruits and vegetables from regular land cultivars (Garcı et al. 2007). The carotenoids and tocols in Codium were found to be the source of vitamins A and E. In C. fragile, all types of tocols were found, with 1617.6 μg g−1 dw for the total tocol content and β-carotene having the high amount (197.9 μg g−1 dw) (Ortiz et al. 2009). Chemical structure of tocols derivated compounds from Codium species is shown in Fig. 3. Meanwhile, C. tomentosum contains vitamins A, C, and E (less than 1.0 mg g−1) and a total carotene content of 15.80 mg per 100 g dw (Celikler et al. 2009).
Structure of tocols found in Codium species, including (a) α-Tocopherol; (b) β-Tocopherol; (c) γ-Tocopherol; (d) δ-Tocopherol (Ortiz et al. 2009)
Bioactivities
From 1990–2021, increasing attention has been paid to the bioactivity and pharmacological properties of the genus Codium (Fig. 4). We found 70 articles that focused on the bioactivity and pharmacological properties of the genus Codium, including immunostimulatory (15.4%), anticoagulant (14.1%), anticancer (12.8%), anti-inflammatory (12.8%), antioxidant (11.5%), antiviral (7.7%), antibacterial (3.8%), antifungal (3.8%), antitumor (3.8%), anti-angiogenic (2.6%), osteoprotective (2.6%), anti-obesity (2.6%), anti-melanogenic (1.3%), anti-sarcopenia (1.3%), antihypertensive (1.3%), neuroprotective (1.3%), and antiprotozoal (1.3%) activities (Fig. 5).
Marine macroalgae contain bioactive compounds such as flavonoids, coumarins, fucosterol, phlorotannin, tocopherols, and nitrogen-containing compounds, including alkaloids, chlorophyll derivatives, amino acids, and amines, which are potential molecules with various pharmacological properties (Celikler et al. 2009; Ali et al. 2015; Gaspar et al. 2020; Meinita et al. 2021, 2022; Harwanto et al. 2022). Several carotenoids, including siphonaxanthin and canthaxanthin, have been reported in C. fragile. Siphonaxanthin is a keto-carotenoid found in siphonaceous green macroalgae, including Codium, that promotes the absorption of available green and blue-green light underwater (Akimoto et al. 2007; Ganesan et al. 2010). Furthermore, siphonaxanthin is known to have beneficial effects on health and to have various other applications (Ganesan et al. 2010; Yim et al. 2021). Codium also contains canthaxanthin, a carotenoid suggested to regulate changes in signaling molecules in C. fragile extracts (Ahn et al. 2021). Two new sulfonoglycosides, codioside E (1) and codioside F (2), have also been identified from the methanol extract of C. dwarkense (Ali et al. 2017).
Research on algal lipids found that loliolide, a ubiquitous monoterpenoid lactone isolated from C. tomentosum, may be used as a neuroprotective agent (Silva et al. 2021). The properties of oleamide, an amide derived from the fatty acid oleic acid of Codium, have been reviewed (Kwon et al. 2001; Moon et al. 2018b). Chemical structure of lipid derivated compounds from Codium species is shown in Fig. 6.
Structure of bioactive compounds from Codium species, including (a) dwarkenoic acid (Ali et al. 2015); (b) siphonaxanthin (Ricketts 1971); (c) canthaxanthin (Rebelo et al. 2020); (d) loliolide (Silva et al. 2021); (e) oleamide (Moon et al. 2018b); (f) sulfonoglycosides: Codioside E and Codioside F (Ali et al. 2017)
Codium has demonstrated significant biological activity both in vitro and in vivo. We review the biological activities attributed specifically to Codium, focusing on those with potential nutraceutical and pharmacological properties. Codium fragile is the most widely studied species in terms of bioactivity. Based on previous research, the polysaccharides and their bioactive compounds in the genus Codium exhibited the highest bioactivity (Table 1).
Immunostimulatory activity
Immunomodulation, which includes immunostimulatory and immunosuppressive effects, is a complicated mechanism that regulates the pathophysiology and etiology of different immune-related disorders. Immunomodulatory substances can be used as immune stimulators to reduce the negative effects of immunosuppressive medicines (Prendergast and Jaffee 2007).Sulfated polysaccharides from marine algae have been shown to have immunostimulatory properties. According to Tabarsa et al. (2013), C. fragile contained sulfated polysaccharide fractions (F1 and F2) in the form of D-galactan with pyruvates and sulfates. The sulfated polysaccharide triggered nitric oxide (NO) production by activating protein and mRNA expression of inducible nitric oxide synthase (iNOS). The sulfated polysaccharides of C. fragile may activate the expression of cytokines inflammatory, including tumor necrosis factor (TNF-α), interleukin (IL)-1, IL-6, and IL-10, as well as promote inflammatory mediators, iNOS and NO production, and protein expression in the RAW 264.7 murine macrophage cell line. As a result, the nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways are also activated by C. fragile sulfated polysaccharides, which seem to stimulate the immune system. Among these two fractions, the F2 fraction, which has a high protein content (14.7%), possessed the most immune-stimulating activity. Furthermore, the F2 fraction can stimulate the gene expression of inflammatory cytokines, including IL-1β, TNF-α, and interferon gamma (IFN-γ) in human cell lines and mouse models (Surayot and You 2017; Yang et al. 2019). The expression of inflammatory cytokines was upregulated in the F2 fraction via the NF-κB and MAPK pathways. The F2 fraction and folic acid-conjugated sulfated polysaccharides significantly increased natural killer cell proliferation and cytotoxicity against HeLa cells (Surayot and You 2017; Li et al. 2020). In addition, in vitro, the F2 fraction was shown to stimulate the expression of the IL-1β gene in head kidney (HK) cells, while in vivo gene expressions of IL-1β and IL-8 were up-regulated in peritoneal cells, HK cells, the liver, the gill, and the spleen. TNF-α, IFN-γ, and lysozyme gene expressions were mainly upregulated, but they differed depending on the tissue type or time point in olive flounder (Paralychthys olivaceus) (Yang et al. 2019). Similar results were obtained in rockfish, Sebastes schlegelii, induced by Edwardsiella tarda, a pathogenic bacterium in fish. This result indicates that IL-1β and IL-6 gene expression was upregulated in the HK of the 0.5% group on day 1, whereas IL-1β gene expression was downregulated in the liver on day 3 (Yang et al. 2021). In addition, sulfated glycoproteins (NF2) and sulfated galactan of C. fragile have immunostimulatory effect in RAW 264.7 cells by activating the NF-κB pathway, thus stimulating the MAPK pathway, including ERK1/2, p38, and JNK1/2, as well as the nuclear translocation of c-JUN and c-FOS (Lee et al. 2010; Tabarsa et al. 2015).
According to Monmai et al. (2020), the expression and production of pro-inflammatory genes and the expression of immune-associated genes were increased by the combination of C. fragile and arachidonic acid via the activation of NF-κB, p-65, and MAPK signaling, including ERK1/2 and p38, which led to the immune-enhancement in RAW 264.7 cells. Another study demonstrated the immune-enhancing effects of anionic macromolecules of C. fragile mixed with red ginseng extract orally administered to cyclophosphamide-treated mice (Kim et al. 2019; Jung and Park 2020). These extracts upregulated the expression of immune-associated genes, thereby inhibiting immune biomarkers by activating the NF-κB and MAPK pathways. These results indicate that polysaccharides and anionic macromolecules extracted from C. fragile are potential sources of immunostimulatory agents.
Anticoagulant activity
In the pharmaceutical industry, there is growing interest in isolating anticoagulant compounds from marine macroalgae. Heparin is a commonly used anticoagulant, but it has some side effects, including thrombocytopenia and spontaneous bleeding (Tardy-poncet et al. 1994). Therefore, it is important to investigate alternatives to anticoagulant agents with fewer heparin-like side effects. Algal polysaccharides have been reported to exhibit heparin-like activity (Faggio et al. 2016). Extracts of C. fragile ssp. atlanticum through low-molecular weight sulfated polysaccharides and high-molecular weight (sulfated) proteoglycans have exhibited anticoagulant properties (Rogers et al. 1990; Jurd et al. 1995). These molecules prolong the thrombin time (TT) and act as antithrombin agents due to potentiation of the activity of the cofactors heparin II and antithrombin III. Furthermore, Athukorala et al. (2007) reported that the crude polysaccharide fraction (CpoF) of C. fragile and Sargassum horneri showed potent anticoagulant properties, with activated partial thromboplastin time (APTT) values of > 300 s. The most potent activity was recorded in the > 30 kDa fraction. The highest molecular weight fraction significantly prolonged clotting times in the APTT and TT assays but had an insignificant effect on the prothrombin time (PT). In addition to prolonging the APTT and PT, C. dwarkense sulfated polysaccharides may reduce the number of microthrombi in the histopathology of the lung, liver, and mesentery with less structural damage in vivo (Golakiya et al. 2017).
In contrast, codiase, a new bifunctional fibrinolytic serine protease isolated from C. fragile, exhibits anticoagulant properties with the prolongation of the APTT and PT, which leads to the inhibition of coagulation factors (Choi et al. 2013). Furthermore, codiase has the potential to block blood-clotting pathways by increasing the anticoagulant action of naturally existing blood factors. An insignificant reduction in fibrinogen levels by codiase may otherwise favor anticoagulation. Codium fragile significantly inhibited platelet activation by downregulating αIIbβ3 signaling and prevented FeCl3-induced arterial thrombus formation without prolonging the bleeding time in vivo (Kim et al. 2021). Finally, the high molecular weight molecules (i.e., polysaccharides and proteoglycans) and codiase of C. fragile could be used as anticoagulant agents.
Anticancer activity
Failure of apoptosis is known to trigger the development of cancer in cells (Shinkai et al. 1996). Apoptosis is a physiological process involving selective cell deletion that regulates the balance between cell proliferation and cell death. Numerous studies have reported the anticancer properties of marine macroalgae. For example, an aqueous extract of C. fragile may inhibit the growth of CT-26 cells and decrease the protein expression of anti-apoptotic Bcl-xL, leading to caspase-3 and caspase-7 activation (Kim et al. 2008). Treatment with C. fragile increases the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and the protein levels of c-caspase-8 and c-caspase-3 by inhibiting cellular FLICE-inhibitory protein (c-FLIP) expression (Hye et al. 2018).
In addition, C. fragile polysaccharides increase the NK cell activation in mice by promoting the activation of bone marrow-derived dendritic cells (BMDCs) in vitro and dendritic cells (DCs) in tumor-bearing mice in vivo (Park et al. 2020b). In an animal model, C. fragile polysaccharides significantly suppressed B16 tumor growth. Moreover, C. fragile polysaccharide treatment inhibited CT-26 cell growth by enhancing anti-cancer immunity mediated by anti-PD-L1 antibodies. According to Wang et al. (2021), C. fragile polysaccharides also reduced Lewis lung carcinoma cell infiltration into the lungs and their anti-tumor growth activity required NK and CD8 T cells. Another study reported that C. fragile polysaccharides inhibited CT-26 and B16 cell infiltration in the lungs (Park et al. 2020a). This study also demonstrated that C. fragile stimulates NK cells. Moreover, C. fragile polysaccharides promote the stimulation of the human peripheral blood DC (PBDC) subset, resulting in T-helper 1 (Th1) cell activation and cytotoxic T lymphocyte (CTL) cell activation, which, in turn, elicits anti-cancer effects (Zhang et al. 2020). Dilshara et al. (2016) evaluated the activity of a methanol extract of C. fragile as a stimulator in human breast cancer MDA-MB-231 cells. They found that that treatment with the methanol extract of C. fragile increased the expression of TNF-α by inhibiting matrix metalloproteinase-9 (MMP-9), further inhibiting NF-κB activity. Cytotoxic effects (IC50 of 150 µM) were also demonstrated in A2058 human melanoma cells treated with C. fragile clerosterol via the upregulation of Bax, downregulation of Bcl-2, and activation of caspases 3 and 9 (Kim et al. 2013). Taken together, C. fragile produces compounds with therapeutic effects against cancer cells by suppressing protein expression and could be used to promote anticancer immunity (Monmai et al. 2019).
Anti-inflammatory activity
Inflammation is a protective response induced by a variety of stimuli, such as physical damage, precursor chemicals, microbial invasion, and immunological responses, in the body (Medzhitov 2008). The infiltration of leukocytes and macrophages is a typical inflammatory reaction. Lipopolysaccharide (LPS) rapidly triggers macrophages and stimulates the secretion of pro-inflammatory cytokines and inflammatory mediators, such as NO and PGE2 via iNOS and COX-2, respectively (Moon et al. 2018b), by upregulating the NF-κB pathway and MAPKs, including the extracellular signal-regulated kinase (ERK)1/2, c-Jun NH2-terminal kinase (JNK), and p38 subfamilies (Sudirman et al. 2019). Currently, alternative anti-inflammatory agents are being identified from marine macroalgae.
It has been found that the extracts of C. fragile, including the aqueous, ethanolic, and methanol extracts, may have anti-inflammatory properties in vitro by inhibiting NO and PGE2 production and reducing inflammatory cytokine levels in LPS-stimulated RAW 264.7 cells, or by inhibiting peptidoglycan (PGN) by blocking NF-κB and MAPK phosphorylation (Han et al. 2010; Yoon et al. 2011; Kang et al. 2012; Ah et al. 2017). Furthermore, an aqueous extract of C. fragile inhibited carrageenan-induced rat paw edema thickness by up to 50% in vivo (Ah et al. 2017). Moon et al (2018b) have also reported that the oleamide from C. fragile may inhibit the inflammatory response in LPS-induced RAW264.7 murine macrophages and reduce carrageenan-induced inflammatory edema in the rat paw model. In addition, the activation of pro-inflammatory proteins, including COX-2, iNOS, and TNF-α, along with pro-inflammatory mediators, including PGE2 and NO, due to the stimulation by ultraviolet B (UVB) irradiation in HaCaT cells decreased after treatment with C. fragile extract. This result also demonstrated that the C. fragile extract reduced oxidative damage, such as lipid peroxidation and/or protein carbonylation, possibly mediated by an increase in antioxidant defense enzymes (Lee et al. 2013).
Antioxidant activity
Antioxidants are important inhibitors of lipid peroxidation; hence, they are used to delay or prevent lipid peroxidation in foods and the oxidation of cellular substrates. All aerobic organisms produce and degrade reactive oxygen species (ROS), including hydroxyl radicals, superoxide anions, hydrogen peroxide (H2O2), and singlet oxygen, resulting in physiological concentrations required for normal cell function or excessive ROS production and subsequently oxidative stress (Nordberg and Arnér 2001). The overproduction of ROS causes damage to cellular macromolecules, such as proteins, DNA, and lipids. Wang et al. (2020) demonstrated that sulfated polysaccharides from C. fragile possessed high toxicity against hydrogen peroxide (H2O2)-induced oxidative stress by reducing intracellular ROS levels, increasing cell viability, and inhibiting apoptosis both in Vero cells and zebrafish in a dose-dependent manner. Furthermore, the aqueous extracts of C. fragile have high scavenging activity against O2–, HO·, H2O2, DPPH free radicals, and ROS (Heo et al. 2005). Another study reported that C. fragile flavonoids with low levels of condensed tannins have fascinating antioxidant profiles (Kolsi et al. 2017a, b). In addition, Celikler et al. (2009) studied the effect of the ethanolic extract of C. tomentosum on chromosomes induced by oxidative stress, and found that it exhibited no genotoxic effects on human lymphocytes in vitro. Finally, the development of antioxidants from marine macroalgae, especially those from C. fragile, is desired for use in the pharmacological industry as a substitute for synthetic antioxidants.
Antiviral activity
Viral treatments address several stages of viral replication, which are broadly defined as entry, replication, shedding, and latency (Kidgell et al. 2019). Enzymatic hydrolysates of C. fragile exhibited significant antiviral activity against the Herpes simplex virus (HSV-1), with an EC50 of 36.5–41.3 μg mL−1 and a multiplicity of infection (MOI) of 0.001 ID50/cells without cytotoxity (1–200 μg mL−1) (Kulshreshtha et al. 2015). Likewise, the extracts from proteases (P1) and carbohydrases (C3) were efficient at a higher MOI, of 0.01 ID50/cells, without cytotoxicity. Selain HSV-1 C. fragile may also inhibit the replication of HSV-2 and the promoted mortality rate in HSV-2-infected mice in vivo (Ohta et al. 2009). Another study demonstrated that siphonaxanthin derived from C. fragile exhibited antiviral activity against the SARS-CoV-2 pseudovirus in HEK293 cells (IC50 = 87.4 µM) (Yim et al. 2021). These results indicate that C. fragile has the potential as a source of novel antiviral agents.
Antibacterial activity
Steroidal glycosides and clerosterol galactoside extracted from C. iyengarii showed moderate in vitro bactericidal activity against Corynebacterium diptheriae, Escherichia coli, Klebsiella pneumoniae, Snigella dysentri, and Staphylococcus aureus (Ali et al. 2010). A significant inhibitory activity against GES-type β-lactamase (GES-22) was observed by the methanol extract of C. tomentosum. Another study on the methanol extract of C. iyengarii exhibited no antibacterial activity against Gram-positive and Gram-negative bacteria. However, it did exhibit good antiviral activity (Rizvi and Shameel, 2004). The variability in activities between species was also demonstrated by Reichelt and Borowitzka (1984) who found that extracts of C. adahaerens, C. muelleri and C. spongiosum showed antibacterial activity against Gram-positive bacteria but not against Gram-negative bacteria, whereas extracts of C. fragile showed no antibacterial activity.
Antifungal activity
Similar to the antibacterial mechanisms, antifungal agents may kill or inhibit fungal pathogens. A previous study on Codium extracts demonstrated that they have antifungal activity. Codium bursa exhibits inhibitory activity against Fusarium spp., Penicillium expansum, Aspergillus flavus, and Rhizophus spp. (Jerkovi et al. 2019). Codium iyengarii exhibited significant antifungal activity against various pathogens, whereas C. shameelii showed weak antifungal activity (Rizvi and Shameel 2004).
Antitumoral activity
Marine macroalgae have been shown to be potential sources of drugs for cancer chemotherapy (Murphy et al. 2014). A sulfated homogalactan from C. isthmocladum showed antitumoral activity by reducing the growth and metastasis of solid tumors without any negative drawbacks. (Bellan et al. 2020). El-Masry et al. (1995) also found that C. sinensis showed antitumoral activity. Zbakh et al. (2020) found that the dichloromethane extract of C. decorticatum effectively reduced tumor cell viability and targeted human cervical cancer cell lines through the apoptotic pathway. Moreover, the dichloromethane extract of C. decorticatum has an anti-proliferative effect by reducing cell viability human of cervical carcinoma HeLa cells through apoptosis in 25.6% of the cells.
Anti-angiogenic activity
Angiogenesis is the physiological process of forming new blood vessels. This process prevents cancer and other related diseases.. The effects of the siphonaxanthin extract from C. fragile and the sulfated galactan extract from C. cylindricum were tested in human umbilical vein endothelial cells (HUVECs) in vitro and in rat aortic rings ex vivo (Matsubara et al. 2003; Ganesan et al. 2010).
Osteoprotective activity
Previous studies have reported the osteoprotective effects of marine macroalgae, including those on osteoporosis and osteoarthritis, both in vitro and in vivo. Osteoporosis is characterized by a decrease in bone mass caused by an imbalance between bone resorption and bone creation, whereas bone homeostasis requires balanced interactions between osteoblasts and osteoclasts (Baek et al. 2016). Meanwhile, the balance in cartilage is disrupted in osteoarthritis, resulting in a substantial increase in inflammatory mediators, ROS, and degradative enzymes, resulting in cartilage degradation and the eventual loss of joint function (Shin et al. 2006). Surget et al. (2017) reported that phenolic compounds from C. fragile may stimulate mineralogenic activity in fish bone-derived cell lines, thereby increasing osteogenic activity by more than 1.5-fold. Moreover, osteoarthritis treatment with an aqueous extract of C. fragile can be relieved by regulating the immune system. The aqueous extract of C. fragile significantly increased the production of nitrite and inflammatory biomarkers (iNOS, MMP-13, ADAMTS-4, and ADAMTS-5) in IL-1β-induced rat primary chondrocytes via interleukin-1β-induced NF-κB signaling activation. Cartilage lesions in the aqueous extract of C. fragile‐treated rats with osteoarthritis exhibited less proteoglycan loss and lower OARSI scores in vivo.
Anti-obesity activity
Obesity has become a global public health issue because it reduces the quality of life of individuals and increases healthcare costs (Maeda 2013). Obesity is defined as the accumulation of body fat. In particular, fat accumulation around internal organs is a major risk factor for various diseases, including type II diabetes, hypertension, dyslipidemia, and cancer (Calle and Thun 2004; Maeda 2015). In recent years, bioactive compounds from marine macroalgae, such as fucoxanthin, alginates, fucoidans, and phlorotannins, have been reported as being potential anti-obesity agents (Wan-Loy and Siew-Moi 2016). Kim et al. (2020) evaluated the anti-obesity effects of C. fragile extracts in mice administered a high-fat diet. They observed that C. fragile extract significantly decreased the body weight and modulated the gut microbiota of the animals by increasing the abundance of beneficial bacteria. It also has been demonstrated that the sulfated polysaccharides of C. fragile effectively decreased the body weight of rats fed a high-fat diet while also protecting hepatic function by increasing the levels of antioxidant enzymes (Kolsi et al. 2017a, b).
Other bioactivities
In addition to the biological effects mentioned above, C. fragile possesses antiprotozoal, antihypertensive, anti-sarcopenia, anti-angiogenic, anti-melanogenic, and neuroprotective activities. Spavieri et al. (2010) isolated a crude extract of C. fragile and demonstrated that it has a high toxicity in protozoan organisms, especially Trypanosoma brucei rhodesiense (IC50 = 8.9 μg mL−1), but it was ineffective against Mycobacterium tuberculosis. The methanol extract from C. fragile exhibits a strong inhibition of the enzyme activity of angiotensin-converting enzyme (ACE) (IC50 = 0.59 mg mL−1), resulting in potent antihypertensive activity (Kolsi et al. 2017a, b). Ahn et al. (2021) showed the potential of a C. fragile extract as a therapeutic agent for sarcopenia management. Sarcopenia is characterized by a loss of skeletal muscle mass and function (Santilli et al. 2014). Ahn et al. (2021) suggested that C. fragile extracts, including LPC, retinoic acid, α-tocopherol, linoleic acid, linolenic acid, and canthaxanthin, enhanced skeletal muscle mass and function by regulating protein synthesis by increasing the phosphorylation of S6K1 and improving the ERRγ-PGC-1α-SIRT1 pathway in myotubes.
Furthermore, siphonaxanthin inhibited HUVEC proliferation and tube formation, while ex vivo treatment effectively suppressed microvessel outgrowth in a dose-dependent manner. In addition, C. fragile extract, at a concentration of 25 µg mL−1, exhibited anti-melanogenic activity through the downregulation of α-melanocyte-stimulating hormone-mediated melanin synthesis in MNT-1 human melanoma cells, as well as through the downregulation of microphthalmia-associated transcription factor, tyrosinase, and tyrosinase-related protein 1. This result, which was produced from a clinical trial, also suggested that the extracellular vesicles of C. fragile may enhance skin brightness. In addition, the neuroprotective activity of loliolide isolated from C. tomentosum can enhance cell viability and reduce oxidative stress, thereby preventing Parkinson’s Disease (Silva et al. 2021).
Conclusion and future directions
In the last three decades studies on the bioactivity and pharmacological properties of the genus Codium have steadily increased. This indicates that the species of Codium have promising potential as sources of various bioactive compounds, such as sulfated polysaccharides, sulfated glycoproteins, dwarkenoic acid, siphonaxanthin, canthaxanthin oleamide, siphonaxanthin, loliode, codioside E, codioside F, α-tocopherol, β-tocopherol, γ-tocopherol, and δ-tocopherol. However, despite the increased research efforts conducted by scientists, to date, only a few products have been developed from Codium species in the pharmaceutical and nutraceutical industries. The present review demonstrates the gap that must be filled in the study of Codium. Two approaches can be applied to develop Codium spp. into products with a high economic value. First, ecological studies should be conducted. Ecological studies include the taxonomy, reproduction, growth, and environmental factors that influence Codium species and their composition (e.g. Marques et al. 2021). This genus is found worldwide as native or invasive species. The occurrence of the Codium species as an invasive species must be considered. A comprehensive study of the reproduction and characteristics of the genus Codium, as well as the dispersal mechanism, needs to be conducted to gain a full understanding of this genus. Furthermore, an aquaculture system for Codium species must be developed and optimized to produce high algal biomass (e.g. Hwang et al. 2008). To date, Codium is little cultivated globally. Second, the identification and isolation of bioactive compounds from species of Codium are needed. Most previously published papers did not go further to isolate and identify the bioactive compounds of Codium. Most studies using crude extracts of Codium species are still in the preliminary stage and the purported active compounds need to be tested in clinical settings. These approaches can be used to develop Codium species into high-value products and maintain their ecological function in marine ecosystems.
References
Ah S, Moon S, Hee Y, Hee S, Park B, Suk M, Kim J, Hwan Y, Kyung D, Sung C (2017) Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed Pharmacother Pharmacother 93:1055–1064
Ahn J, Kim JM, Ahyoung Y, Ahn J, Ha TY, Jung CH, Seo HD, Jang YJ (2021) Identifying Codium fragile extract components and their effects on muscle weight and exercise endurance. Food Chem 353:129463
Akimoto S, Tomo T, Naitoh Y, Otomo A, Murakami A, Mimuro M (2007) Identification of a new excited state responsible for the in vivo unique absorption band of siphonaxanthin in the green alga Codium fragile. J Phys Chem B 111:9179–9181
Ali L, Al-Kharusi L, Al-Harrasi A (2017) Two new sulfonoglycolipids from the green alga Codium dwarkense. Nat Prod Commun 12:583–585
Ali L, Khan AL, Al-Kharusi L, Hussain J, Al-Harrsi A (2015) New α-glucosidase inhibitory triterpenic acid from marine macro green alga Codium dwarkense Boergs. Mar Drugs 13:4344–4356
Ali MS, Saleem M, Yamdagni R, Ali MA (2010) Steroid and antibacterial steroidal glycosides from marine green alga Codium iyengarii Borgesen. Nat Prod Lett 16:407–413
Aliya R, Shameel M (1993) Phycochemical examination of three species of Codium (Bryopsidophyceae). Bot Mar 36:371–376.
Allan EL, Ambrose ST, Richoux NB, Froneman PW, Town C (2010) Determining spatial changes in the diet of nearshore suspension-feeders along the South African coastline : Stable isotope and fatty acid signatures. Estuar Coast Shelf Sci 87:463–471
An JW, Oo, Nam KW (2015) New record of Codium lucasii ( Bryopsidales, Chlorophyta ) in Korea. J Ecol 38:647–654
Armitage CS, Sjøtun K (2016) Codium fragile in Norway : subspecies identity and morphology. Bot Mar 59:439–450
Athukorala Y, Lee K, Kim S, Jeon Y (2007) Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol 98:1711–1716
Baek SH, Jeong SC, Jeong YT, Yoon YD, Kim OH, Oh BC, Jung JW, Kim JH (2016) Osteoprotective effects of polysaccharide-enriched Hizikia fusiforme processing byproduct in vitro and in vivo models. 19: 3646
Bellan DL, Biscaia SMP, Rossi GR, Cristal AM, Gonçalves JP, Oliveira CC, Simas FF, Sabry DA, Rocha HAO, Franco CRC, Chammas R, Gillies RJ, Trindade ES (2020) Green does not always mean go : A sulfated galactan from Codium isthmocladum green seaweed reduces melanoma metastasis through direct regulation of malignancy features. Carbohydr Polym 250:116869
Ben-Avraham Z (1971) Accumulation of stones on beaches by Codium fragile. Limnol Oceanogr 16:553–554
Benson EE, Rutter JC, Cobb AH (1983) Seasonal variation in frond morphology and chloroplast physiology of the intertidal alga Codium fragile (Suringar) Hariot. New Phytol 95:569–580
Bilan MI, Vinogradova EV, Shashkov AS, Usov AI (2006) Isolation and preliminary characterization of a highly pyruvylated galactan sulfate from Codium yezoense (Bryopsidales, Chlorophyta). Bot Mar 49:259–262
Bilan MI, Vinogradova EV, Shashkov AS, Usov AI (2007) Structure of a highly pyruvylated galactan sulfate from the pacific green alga Codium yezoense (Bryopsidales, Chlorophyta). Carbohydr Res 342:586–596
Bulleri F, Airoldi L, Branca G, Abbiati M (2006) Positive effects of the introduced green alga, Codium fragile ssp. tomentosoides, on recruitment and survival of mussels. Mar Biol 148:1213–1220
Cabrita ARJ, Maia MRG, Oliveira HM, Sousa-Pinto I, Almeida AA, Pinto E, Fonseca AJM (2016) Tracing seaweeds as mineral sources for farm-animals. J Appl Phycol 28:3135–3150
Calle EE, Thun MJ (2004) Obesity and cancer. Oncogene 23:6365–6378
Carneiro RF, Duarte PL, Chaves RP, Roberta S, Feitosa RR, Sousa BL, Alves AWS, Vasconcelos MA, Rocha BAM, Teixeira EH, Sampaio AH, Nagano CS (2020) New lectins from Codium isthmocladum Vickers show unique amino acid sequence and antibiofilm effect on pathogenic bacteria. J Appl Phycol 32:4263–4276
Celikler S, Vatan O, Yildiz G, Bilaloglu R (2009) Evaluation of anti-oxidative, genotoxic and antigenotoxic potency of Codium tomentosum Stackhouse ethanolic extract in human lymphocytes in vitro. Food Chem Toxicol 47:796–801
Choi JH, Sapkota K, Park SE, Kim S, Kim SJ (2013) Thrombolytic, anticoagulant and antiplatelet activities of codiase, a bi-functional fibrinolytic enzyme from Codium fragile. Biochimie 95:1266–1277
Churchill AC, Moeller HW (1972) Seasonal patterns of reproduction in New York Populations of Codium fragile (Sur.) Hariot subsp. tomentosoides (Van Goor) Silva. J Phycol 8:147–152
Ciancia M, Quintana I, Vizcargüénaga MI, Kasulin L, de Dios A, Estevez JM, Cerezo AS (2007) Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Biol Macromol 41:641–649
Circuncis AR, Catarino MD, Cardoso SM, Silva AMS (2018) Minerals from macroalgae origin : health benefits and risks for consumers. Mar Drugs 16:400
da Costa E, Melo T, Moreira ASP, Alves E, Domingues P, Calado R, Abreu MH, Domingues RM (2015) Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res 12:388–397
de Oliveira-Carvalho M, de F, Oliveira MC, Pereira SMB, Verbruggen H, (2012) Phylogenetic analysis of Codium species from Brazil, with the description of the new species C. pernambucensis (Bryopsidales, Chlorophyta). Eur J Phycol 47:355–365
Dembitsky VM, Hanus LO (2003) Variability of the fatty acids of the marine green algae belonging to the genus Codium ezankova. 31:1125–1145
Dilshara MG, Jayasooriya RGPT, Kang CH, Choi YH, Kim GY (2016) Methanol extract of Codium fragile inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 and invasiveness of MDA-MB-231 cells by suppressing nuclear factor-κB activation. Asian Pac J Trop Med 9:535–541
Dromgoole FI (1975) Occurrence of Codium fragile subspecies tomentosoides in New Zealand waters. N Z J Mar Freshw Res 9:257–264
Drouin A, Mckindsey CW, Johnson LE (2011) Higher abundance and diversity in faunal assemblages with the invasion of Codium fragile ssp. fragile in eelgrass meadows. Mar Ecol Prog Ser 424:105–117
El-Masry MH, Mostafa MH, Ibrahim AM, El-Naggar MMA (1995) Marine algae that display anti-tumorigenic activity against Agrobacterium tumefaciens. Microbiol Lett 128:151–156
El-Said GF, El-Sikaily A (2013) Chemical composition of some seaweed from Mediterranean Sea coast. Egypt Environ Monit Assess 185:6089–6099
Estevez JM, Fernandez PV, Kasulin L, Dupree P, Ciancia M (2009) Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 19:212–228
Faggio C, Pagano M, Dottore A, Genovese G, Morabito M (2016) Evaluation of anticoagulant activity of two algal polysaccharides. Nat Prod Res 30:1934–1937
Farias EH, Pomin VH, Valente AP, Nader HB, Rocha HA, Mourão PA (2008) A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 18:250–259
Fernández PV, Arata PX, Ciancia M (2014) Polysaccharides from Codium species: Chemical structure and biological activity. their role as components of the cell wall. Adv Bot Res 71:253–278
Fernández PV, Estevez JM, Cerezo AS, Ciancia M (2012) Sulfated β-d-mannan from green seaweed Codium vermilara. Carbohydr Polym 87:916–919
Fernández PV, Raffo MP, Alberghina J, Ciancia M (2015) Polysaccharides from the green seaweed Codium decorticatum. Structure and cell wall distribution. Carbohydr Polym 117:836–844
Fernández PV, Quintana I, Cerezo AS, Ciancia M (2013) Anticoagulant activity of a unique sulfated pyranosic (1,3)-arabinan through direct interaction with thrombin. J Biol Chem 288:223–233
Ganesan P, Matsubara K, Ohkubo T, Tanaka Y, Noda K, Sugawara T, Hirata T (2010) Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 17:1140–1144
Garcı MN, Pereira AC, Leets I, Quiroga MF (2007) High iron content and bioavailability in humans from four species of marine algae. Nutr Physiol Metab Nutr Interact 137:2691–2695
Gaspar F, Avaro MG, Commendatore MG, Arce L, de Vivar MED (2020) The macroalgal ensemble of Golfo Nuevo ( Patagonia, Argentina ) as a potential source of valuable fatty acids for nutritional and nutraceutical purposes. Algal Res 45:101726
Gisone P, Robello E, Sanjurjo J, Dubner D, Pérez MDR, Michelin S, Puntarulo S (2006) Reactive species and apoptosis of neural precursor cells after γ-irradiation. Neurotoxicology 27:253–259
Goecke F, Hernández V, Bittner M, González M, Becerra J, Silva M (2010) Fatty acid composition of three species of Codium (Bryopsidales, Chlorophyta) in Chile. Rev Biol Mar Oceanogr 45:
Golakiya HN, Naik VN, Hirapara HN, Modu KH, Goswami AP, Tripathi C (2017) Evaluation of anticoagulant effect of sulfated polysaccharide (Sps) from Codium dwarkense Børgesen in κ-carrageenan induced hypercoagulable state in Wistar albino rats. Acta Pol Pharm - Drug Res 74:987–994
González AV, Chacana ME, Silva PC (2012) Codium bernabei sp. nov. (Bryopsidales, Chlorophyta), a coalescing green seaweed from the coast of Chile. Phycologia 51:666–671
Guiry MD, Guiry GM (2022) AlgaeBase. World-wide electronic publication. National University of Ireland. http://www.algaebase.org
Han SH, Kim YG, Lee SH, Park CB, Han SW, Jang HJ, Lee HJ, Park SC, Kim HS, Lee YS, Kwon DY (2010) Anti-inflammatory activity of Codium fragile in macrophages induced by peptidoglycan. Nat Prod Sci 16:153–158
Hanisak MD (1979) Growth patterns of Codium fragile ssp. tomentosoides in response to temperature, irradiance, salinity, and nitrogen source. Mar Biol 50:319–332
Harwanto D, Negara BFSP, Tirtawijaya G, Meinita MDN, Choi JS (2022) Evaluation of toxicity of crude phlorotannins and phloroglucinol using different model organisms. Toxins (Basel) 14:312
Heo SJ, Cha SH, Lee KW, Cho SK, Jeon YJ (2005) Antioxidant activities of chlorophyta and phaeophyta from Jeju island. Algae 20:251–260
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: Functional food applications and legislation. J Appl Phycol 23:543–597
Houchi S, Mahdadi R, Khenchouche A, Song J, Zhang W, Pang X, Zhang L, Sandalli C, Du G (2019) Investigation of common chemical components and inhibitory effect on GES-type β-lactamase (GES22) in methanolic extracts of Algerian seaweeds. Microb Pathog 126:56–62
Hubbard CB, Garbary DJ (2002) Morphological variation of Codium fragile (Chlorophyta) in eastern Canada. Bot Mar 45:476–485
Hwang EK, Baek JM, Park CS (2008) Cultivation of the green alga, Codium fragile (Suringar) Hariot, by artificial seed production in Korea. J Appl Phycol 20:469–475
Hwang EK, Park CS (2020) Seaweed cultivation and utilization of Korea. Algae 35:107–121
Hye S, Lim J, Jeong S, Ram B, Jin Y, Jee M, Kyeong H, Jeong YA, Yeong D, Gyeom B, You S, Cheul S, Lee D (2018) Biochemical and biophysical research communications Codium fragile F2 sensitize colorectal cancer cells to TRAIL-induced apoptosis via c-FLIP ubiquitination. Biochem Biophys Res Commun 508:1–8
Jang B, Chung H, Jung H, Song H, Park E, Choi HS, Jung K, Choe H, Yang S, Oh E (2021) Molecules and cells extracellular vesicles from Korean Codium fragile and Sargassum fusiforme negatively regulate melanin synthesis. Mol Cells 44:736–745
Jerković I, Kranjac M, Marijanović Z, Šarkanj B, Cikoš AM, Aladić K, Pedisić S, Jokić S (2019) Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids and insight into its antifungal activity. Molecules 24:842
Jung S, Park WJ (2020) Co-immunomodulatory activities of anionic macromolecules extracted from Codium fragile with red ginseng extract on peritoneal macrophage of immune-suppressed mice. J Microbiol Biotechnol 30:352–358
Jurd KM, Rogers DJ, Blunden G, McLellan DS (1995) Anticoagulant properties of sulphated polysaccharides and a proteoglycan from Codium fragile ssp. atlanticum. J Appl Phycol 7:339–345
Kang CH, Choi YH, Park SY, Kim GY (2012) Anti-inflammatory effects of methanol extract of Codium fragile in lipopolysaccharide-stimulated RAW 264.7 cells. J Med Food 15:44–50
Kang YH, Shin JA, Kim MS (2008) A preliminary study of the bioremediation potential of Codium fragile applied to seaweed integrated multi-trophic aquaculture (IMTA) during the summer. J Appl Phycol 20:183–190
Khan MNA, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, Hong YK (2008) Anti-inflammatory activities of methanol extracts from various seaweed species. J Environ Biol 29:465–469
Khotimchenko SV (2003) Fatty acids of species in the genus Codium. Bot Mar 46:456–460
Kidgell JT, Magnusson M, de Nys R, Glasson CRK (2019) Ulvan: A systematic review of extraction, composition and function. Algal Res 39:101422
Kim AD, Lee Y, Kang SH, Kim GY, Kim HS, Hyun JW (2013) Cytotoxic effect of clerosterol isolated from Codium fragile on A2058 human melanoma cells. Mar Drugs 11:418–430
Kim J, Choi JH, Oh T, Ahn B, Unno T (2020) Codium fragile ameliorates high-fat diet-induced metabolism by modulating the gut microbiota in mice. Nutrients 12:1848
Kim JE, Monmai C, Rod-in W, Jang A, You S, Lee S, Park WJ (2019) Immune enhancement effects of Codium fragile anionic macromolecules combined with red ginseng extract in immune-suppressed mice. J Microbiol Biotechnol 29:1361–1368
Kim KN, Kim SH, Kim WS, Kang SM, Lee KW, Wook JL, Park SY, Kim SK, Jeon YJ (2008) Antitumor activities of sea staghorn (Codium fragile) against CT-26 cells. Food Sci Biotechnol 17:976–982
Kim TI, Kim Y, Kim K (2021) Extract of seaweed Codium fragile inhibits integrin αIIbβ3-induced outside-in signaling and arterial thrombosis. Front Pharmacol 12:685948
Kolsi RBA, Jardak N, Hadjkacem F, Chaaben R, Feki AE, Rebai T, Jamoussi K, Fki L, Belghith H, Belghith K (2017a) Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats. Int J Biol Macromol 102:119–129
Kolsi RBA, Ben SH, Hamza A, El Feki A, Allouche N, El Feki L, Belguith K (2017b) Characterization and evaluating of antioxidant and antihypertensive properties of green alga (Codium fragile) from the coast of Sfax. J Pharmacogn Phytochem 6:186–191
Kolsi RBA, Jardak N, Hajkacem F, Chaaben R, Jribi I, Feki AE, Rebai T, Jamoussi K, Fki L, Belghith H, Belghith K (2017c) Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats. Int J Biol Macromol 102:119–129
Kulshreshtha G, Burlot A, Marty C, Critchley A, Hafting J, Bedoux G, Bourgougnon N, Prithiviraj B (2015) Enzyme-assisted extraction of bioactive material from Chondrus crispus and Codium fragile and its effect on Herpes simplex Virus (HSV-1). Mar Drugs 13:558–580
Kwon H, Hwang S, Han J, Kim C, Rho J, Shin J (2001) Production of oleamide, a functional lipid, by Streptomyces sp. KK90378. Microbiol Biotechnol 11:1018–1023
Lafarga T, Acién-Fernández FG, Garcia-Vaquero M (2020) Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res 48:101909
Lee C, Hwan G, Mi E, Kim B, Park C, Jang J (2013) Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 86:54–63
Lee J-B, Hayashi K, Maeda M, Hayashi T (2004) Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med 70:813–817
Lee J, Ohta Y, Hayashi K, Hayashi T (2010) Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr Res 345:1452–1454
Li C, Palanisamy S, Talapphet N, Cho M, You S (2020) Preparation and characterization of folic acid conjugated sulfated polysaccharides on NK cell activation and cellular uptake in HeLa cells. Carbohydr Polym 154:117250
Li N, Mao W, Yan M, Liu X, Xia Z, Wang S, Xiao B, Chen C, Zhang L, Cao S (2015) Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr Polym 121:175–182
Lopes D, Melo T, Rey F, Meneses J, Monteiro FL, Helguero LA, Abreu MH, Lillebø AI, Calado R, Domingues MR (2020) Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 25:3883
Love J, Percival E (1964) The polysaccharides of the green seaweed Codium fragile. Part II . The water-soluble sulphated polysaccharides. J Chem Soc 3338–3345
Maeda H (2013) Anti-obesity and anti-diabetic activities of algae. In: Domínguez H (ed) Functional ingredients from algae for foods and nutraceuticals. Woodhead Publishing , Oxford, pp 453–472
Maeda H (2015) Nutraceutical effects of fucoxanthin for obesity and diabetes therapy : A review. J Oleo Sci 64:125–132
Malea P, Chatziapostolou A, Kevrekidis T (2015) Trace element seasonality in marine macroalgae of different functional-form groups. Mar Environ Res 103:18–26
Marques R, Cruz S, Calado R, Lillebø A, Abreu H, Pereira R, Pitarma B, da Silva JM, Cartaxana P (2021) Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum. J Appl Phycol 33:471–480
Matsubara K, Matsuura Y, Bacic A, Liao M, Hori K, Miyazawa K (2001) Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Biol Macromol 28:395–399
Matsubara K, Mori M, Matsumoto H, Hori K, Miyazawa K (2003) Antiangiogenic properties of a sulfated galactan isolated from a marine green alga, Codium cylindricum. J Appl Phycol 15:87–90
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Meinita MDN, Harwanto D, Choi JS (2022) Seaweed exhibits therapeutic properties against chronic diseases: an overview. Appl Sci 12:2638
Meinita MDN, Harwanto D, Tirtawijaya G, Negara BFSP, Sohn JH, Kim JS, Choi JS (2021) Fucosterol of marine macroalgae: Bioactivity, safety and toxicity on organism. Mar Drugs 19:545
Monmai C, Rod-in W, Jang A, Lee S, Jung SK, You S, Park WJ (2020) Immune-enhancing effects of anionic macromolecules extracted from Codium fragile coupled with arachidonic acid in RAW264.7 cells. PLoS ONE 15:e0239422
Monmai C, You S, Park WJ (2019) Immune-enhancing effects of anionic macromolecules extracted from Codium fragile on cyclophosphamide-treated mice. PLoS ONE 14:e0211570
Moon SM, Lee SA, Han SH, Park BR, Choi MS, Kim JS, Kim SG, Kim HJ, Chun HS, Kim DK, Kim CS (2018a) Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed Pharmacother 97:264–270
Moon SM, Lee SA, Hong JH, Kim JS, Kim DK, Kim CS (2018b) Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int Immunopharmacol 56:179–185
Moreda-Pineiro A, Pena-Vasquez E, Bermejo-Barrera P (2012) Significance of the presence of trace and ultratrace elements in seaweeds. In: Kim S-K (ed) Handbook of Marine Macroalgae: Biotechnology and Applied Phycology. John Wiley & Sons Ltd, New York, pp 116–169
Muha TP, Skukan R, Borrell YJ, Rico JM, Garcia de Leaniz C, Garcia-Vazquez E, Consuegra S (2019) Contrasting seasonal and spatial distribution of native and invasive Codium seaweed revealed by targeting species - specific eDNA. Ecol Evol 9:8567–8579
Murphy C, Hotchkiss S, Worthington J, McKeown SR (2014) The potential of seaweed as a source of drugs for use in cancer chemotherapy. J Appl Phycol 26:2211–2264
Nanba N, Kado R, Ogawa H, Nakagawa T, Sugiura Y (2005) Effects of irradiance and water flow on formation and growth of spongy and filamentous thalli of Codium fragile. Aquat Bot 81:315–325
Neto AIA, Prestes ACL, Álvaro NV, Resendes R, Neto RMA, Tittley I, Moreu I (2020) Marine algal flora of Pico Island, Azores. Biodivers Data J 8:1–32
Nordberg J, Arnér ESJ (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312
Ohta Y, Lee J-B, Hayashi K, Hayashi T (2009) Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol Pharm Bull 32:892–898
Ortiz J, Uquiche E, Robert P, Romero N, Quitral V, Llantén C (2009) Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur J Lipid Sci Technol 111:320–327
Park HB, Hwang J, Zhang W, Go S, Kim J, Choi I, You SG, Jin JO (2020a) Polysaccharide from Codium fragile induces anti-cancer immunity by activating natural killer cells. Mar Drugs 18:626
Park H, Lim SM, Hwang J, Zhang W, You S, Jin JO (2020b) Cancer immunotherapy using a polysaccharide from Codium fragile in a murine model model. Oncoimmunology 9:1772663
Pereira H, Barreira L, Figueiredo F, Custódio L, Vizetto-Duarte C, Polo C, Rešek E, Aschwin E, Varela J (2012) Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar Drugs 10:1920–1935
Prendergast GC, Jaffee EM (2007) Cancer immunologists and cancer biologists : Why We didn’t talk then but need to now. Perspect Cancer Res 67:3500–3505
Prince JS, Trowbridge CD (2004) Reproduction in the green macroalqa Codium (Chlorophyta): Characterization of gametes. Bot Mar 47:461–470
Provan J, Booth D, Todd NP, Beatty GE, Maggs CA (2008) Tracking biological invasions in space and time : elucidating the invasive history of the green alga Codium fragile using old DNA. Divers Distrib 14:343–354
Provan J, Murphy S, Maggs CA (2005) Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides. Mol Ecol 14:189–194
Rebelo BA, Farrona S, Ventura MR, Abranches R (2020) Canthaxanthin, a red-hot carotenoid: applications, synthesis, and biosynthetic evolution. Plants 9:1039
Reichelt JL, Borowitzka MA (1984) Antibiotics from algae: results of a large scale screening programme. Hydrobiologia 116:158–168
Rengasamy KR, Amoo SO, Aremu AO, Stirk WA, Gruz J, Šubrtová M, Doležal K, Van Staden J (2015) Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J Appl Phycol 27:1599–1605
Rey F, Cartaxana P, Melo T, Calado R, Pereira R, Abreu H, Domingues P, Cruz S, Domingues MR (2020) Domesticated populations of Codium tomentosum display lipid extracts with lower seasonal shifts than conspecifics from the wild-relevance for biotechnological applications of this green seaweed. Mar Drugs 18:188
Ricketts TR (1971) The Structure of siphonein and siphonaxanthin from Codium fragile. Phytochemistry 10:155–160
Rizvi MA, Shameel M (2004) Studies on the bioactivity and elementology of marine algae from the coast of Karachi, Pakistan. Phyther Res 18:865–872
Rodrigues D, Freitas AC, Pereira L, Rocha-Santos TAP, Vasconcelos MW, Roriz M, Rodríguez-Alcalá LM, Gomes AMP, Duarte AC (2015) Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem 183:197–207
Rogers DJ, Jurd KM, Blunden G, Paoletti S, Zanetti F (1990) Anticoagulant activity of a proteoglycan in extracts of Codium fragile ssp. atlanticum. J Appl Phycol 2:357–361
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Sabry DA, Corderio SL, Silva CHF, Farias EHC, Sassaki GL, Nader HB, Rocha HAO (2019) Pharmacological prospection and structural characterization of two purified sulfated and pyruvylated homogalactans from green algae Codium isthmocladum. Carbohydr Polym 222:115010
Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11:177–180
Scheibling RE, Gagnon P (2006) Competitive interactions between the invasive green alga Codium fragile ssp. tomentosoides and native canopy-forming seaweeds in Nova Scotia (Canada). Mar Ecol Prog Ser 325:1–14
Schmid M, Kraft LGK, Van Der LLM, Kraft GT, Virtue P, Nichols PD, Hurd CL (2018) Southern Australian seaweeds : A promising resource for omega-3 fatty acids. Food Chem 265:70–77
Schmidt AL, Scheibling RE (2006) A comparison of epifauna and epiphytes on native kelps (Laminaria species) and an invasive alga (Codium fragile ssp. tomentosoides) in Nova Scotia, Canada. Bot Mar 49:315–330
Schmidt AL, Scheibling RE (2005) Population dynamics of an invasive green alga, Codium fragile subsp. tomentosoides, in tidepools on a rocky shore in. Ecoscience 12:403–411
Senthilkumar D, Jayanthi S (2016) Partial characterization and anticancer activities of purified glycoprotein extracted from green seaweed Codium decorticatum. J Funct Foods 25:323–332
Seo U, Kang H, Yoon K, An Y (2019) Analysis of dietary fiber, mineral content and fatty acid composition in cheonggak (Codium fragile). Korean J Food Nutr 32:328–334
Shameel M (1990) Phycochemical studies on fatty acids from certain seaweeds. Bot Mar 33:429–432
Shin H, Hwang HJ, Kang KJ, Lee BH (2006) An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharm Res 29:165–171
Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, Kitagawa I (1996) Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Japanese J Cancer Res 87:357–362
Siddhanta AK, Shanmugam M, Mody KH, Goswami AM, Ramavat BK (1999) Sulphated polysaccharides of Codium dwarkense Boergs. from the west coast of India : chemical composition and blood anticoagulant activity. Int J Biol Macromol 26:151–154
Silva J, Alves C, Martins A, Sim M, Guedes M, Rehfeldt S, Pinteus S, Gaspar H, Goettert I, Alfonso A, Pedrosa R (2021) Loliolide, a new therapeutic option for neurological diseases ? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int J Mol Sci 22:1–21
Skrzypczyk VM, Hermon KM, Norambuena F, Turchini GM, Keast R, Bellgrove A (2018) Is Australian seaweed worth eating ? Nutritional and sensorial properties of wild-harvested Australian versus commercially available seaweeds. J Appl Phycol 31:706–724
Spavieri J, Kaiser M, Casey R, Hingley-Wilson S, Lalvani A, Blunden G, Tasdemir D (2010) Antiprotozoal, Antimycobacterial and cytotoxic potential of some british green algae. Phyther Res 24:1095–1098
Stirk WA, Reinecke DL, van Staden J (2007) Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J Appl Phycol 19:271–276
Sudirman S, Chang HW, Chen CK, Kong ZL (2019) A dietary polysaccharide from: Eucheuma cottonii downregulates proinflammatory cytokines and ameliorates osteoarthritis-associated cartilage degradation in obese rats. Food Funct 10:5697–5706
Surayot U, You S (2017) Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int J Biol Macromol 98:117–124
Surget G, Roberto VP, Le Lann K, Mira S, Guérard F, Laizé V, Poupart N, Cancela ML, Stiger-Pouveran V (2017) Marine green macroalgae : a source of natural compounds with mineralogenic and antioxidant activities. J Appl Phycol 29:575–584
Tabarsa M, Karnjanapratum S, Cho M, Kim JK, You S (2013) Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int J Biol Macromol 59:1–12
Tabarsa M, Park GM, Shin IS, Lee E, Kim JK, You S (2015) Structure-activity relationships of sulfated glycoproteins from Codium fragile on nitric oxide releasing capacity. Mar Biotechnol 17:266–276
Tardy-Poncet B, Tardy B, Grelac F, Reynaud J, Mismetti P, Bertrand JC, Guyotat D (1994) Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am J Hematol 45:252–257
Thomsen MST, McGlathery KJ, Tyler AC (2006) Macroalgal distribution patterns in a shallow, soft-bottom lagoon, with emphasis on the nonnative Gracilaria vermiculophylla and Codium fragile. Estuaries Coasts 29:465–473
Valentão P, Trindade P, Gomes D, de Pinho PG, Mouga T, Andrade PB (2010) Codium tomentosum and Plocamium cartilagineum : Chemistry and antioxidant potential. Food Chem 119:1359–1368
Verbruggen H (2014) Morphological complexity, plasticity, and species diagnosability in the application of old species names in DNA-based taxonomies. J Phycol 50:26–31
Verbruggen H, Costa JF (2015) Molecular survey of Codium species diversity in southern Madagascar. Cryptogam Algol 36:171–187
Verbruggen H, Leliaert F, Maggs CA, Shimada S, Schils T, Provan J, Booth D, Murphy S, De Clerck O, Littler DS, Littler MM, Coppejans E (2007) Species boundaries and phylogenetic relationships within the green algal genus Codium (Bryopsidales) based on plastid DNA sequences. Mol Phylogenet Evol 44:240–254
Verbruggen H, Pauly K, De Clerck O (2012) The new species Codium recurvatum from Tanzania. Eur J Phycol 47:216–222
Vilà M, Basnou C, Pyšek P, Josefsson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, Roy D, Hulme PE, DAISIE partners, (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8:135–144
Wan-Loy C, Siew-Moi P (2016) Marine algae as a potential source for anti-obesity agents. Mar Drugs 14:222
Wang L, Wang X, Wu H, Liu R (2014) Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar Drugs 12:4984–5020
Wang L, Young J, Geon J, Jayawardena TU, Kim Y, Young J, Fu X, Jeon Y (2020) Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res 48:101891
Wang Y, An EK, Kim SJ, You S, Jin JO (2021) Intranasal administration of Codium fragile polysaccharide elicits anti-cancer immunity against Lewis Lung Carcinoma. Int J Mol Sci 22:10608
Woo LH, Sook KM (2015) Species delimitation in the green algal genus Codium (Bryopsidales) from Korea using DNA barcoding. Acta Oceanol Sinica 34:114–124
Xu X, Tran VH, Kraft JG, Beardall J (1998) Fatty acids of six Codium species from southeast Australia. Phytochemistry 48:1335–1339
Yan S, Pan C, Yang X, Chen S, Qi B, Huang H (2021) Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: Structural characterization and antioxidant activity. Int J Biol Macromol 182:129–135
Yang Y, Lim J, Li C, Lee S, Hong S (2021) Effects of sulfated polysaccharides isolated from Codium fragile on inflammatory cytokine gene expression and Edwardsiella tarda infection in rockfish, Sebastes schlegelii. Fish Shellfish Immunol 112:125–134
Yang Y, Park J, You SG, Hong S (2019) Immuno-stimulatory effects of sulfated polysaccharides isolated from Codium fragile in olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol 87:609–614
Yasmeen A, Ibrahim M, ul Hasan MM, Jilani T, Shafique S, Rasheed M (2021) Phycochemical analyses and pharmacological activities of seven macroalgae of Arabian Sea (Northern coast line). Pak J Pharm Sci 34:963–969
Yim S, Kim I, Warren B, Kim J, Jung K, Ku B (2021) Antiviral activity of two marine carotenoids against SARS-CoV-2 virus entry in silico and in vitro. Int J Mol Sci 22:6481
Yoon HD, Jeong EJ, Choi JW, Lee MS, Park MA, Yoon NY, Kim YK, Cho DM, Kim JI, Kim HR (2011) Anti-inflammatory effects of ethanolic extracts from Codium fragile on LPS-stimulated RAW 264.7 macrophages via nuclear factor κB inactivation. Fish Aquat Sci 14:267–274
Zbakh H, Salhi G, Bochkov V, Ciudad CJ, Noé V, Riadi H (2020) Insight on the anti-inflammatory and antitumor activities of extracts from the marine green alga Codium decorticatum. Eur J Integr Med 37:101170
Zhang W, Hwang J, Park H, Lim S, Go S, Kim J, Choi I, You S, Jin JO (2020) Human peripheral blood dendritic cell and T cell activation by Codium fragile polysaccharide. Mar Drugs 18:535
Zheng T, Liu C, Yang J, Liu Q, Li J (2013) Hijiki seaweed (Hizikia fusiformis): Nutritional value, safety concern and arsenic removal method. Adv Mater Res 634–638:1247–1252
Acknowledgements
This research was supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (PJT200885). The research was also supported by the Ministry of Oceans and Fisheries and Busan Metropolitan City through the Capacity Building Project for the Development of Busan Seafood Industry-Based Complex. We also thank to Silla University, Jenderal Soedirman University and Diponegoro University for the international research collaboration.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meinita, M.D.N., Harwanto, D. & Choi, JS. A concise review of the bioactivity and pharmacological properties of the genus Codium (Bryopsidales, Chlorophyta). J Appl Phycol 34, 2827–2845 (2022). https://doi.org/10.1007/s10811-022-02842-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02842-8