Abstract
Oat is a worldwide cultivated crop with nutritional, ecological and economic value. The hardness of oat is closely related to the damage to the grain during harvesting, washing, threshing and hulling, and has an important impact on the processing quality and eating quality of oats. Vromindolines are a group of proteins specific to the Avena genus and are responsible for the softness of oat endosperm. Avena nuda is an allohexaploid, and is an important grain and feed crop in China. Vromindolines have been studied in the genus Oat, but have not been reported in Avena nuda. In this study, we performed paraffin sectioning and scanning electron microscopy analysis on oat kernels with different hardness, and the sequence characteristics, allelic variation, expression patterns and subcellular localization of Vromindoline-1 and Vromindoline-3 genes in Avena nuda were also analyzed. The results showed that the cell structure and ultrastructure of soft oat variety grains are significantly different from those of hard oats variety. PCR amplification and sequence analysis showed that the lengths of AnVin-1 and AnVin-3 genes were 444 and 429 bp, respectively, and AnVin-1 existed in the oat A, C and D genomes. Expression analysis in different tissues showed that AnVin-1A, AnVin-1C, AnVin-1D and AnVin-3C were expressed to varying degree in roots, stems and leaves. Expression analysis at the panicle developmental stage showed that the expression levels of these four genes first increased and then decreased, with the highest expression levels at 14 days after pollination. In addition, the expression levels of AnVin-1C and AnVin-3C in soft oat variety were higher than those in hard oats at 14 DAP. Among the single nucleotide polymorphisms among 18 AnVin-1C and 9 AnVin-3C gene sequences, AnVin-1C6 and AnVin-3C2 genes had three haplotypes in a oat natural population. Based on the allelic variation sites of AnVin-1C6, a cleavage amplified polymorphic sequence marker was established, which explained 1.44% of the variation in hardness. The above results of this study indicated that AnVin-1 and AnVin-3 genes were involved in kernel development and might affect kernel firmness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the cereal crops, oat is characterized by a soft endosperm texture, which has a negative impact on the industrial processing of oats, such as reduced oatflake yield (Alfieri et al. 2014). The single kernel characterization system (SKCS) is usually used to determine the hardness value of oat (Gazza et al. 2015) and has certain advantages in measuring hardness (Jianghong et al. 2020). Our previous research compared the accuracy of SKCS and Texture Analyzer (TA), the results showed that TA method was more suitable in detecting the kernel hardness of A. nuda (Jianghong et al. 2021).
The endosperm texture is the result of the combined effect of the biomolecular composition of the grain (Chunqing 1992). Wheat kernel hardness depends on the cohesion between the starch granules, the protein matrix, and the continuity of the protein matrix that coats the starch granules (Greenwell & Schofield 1986). Both genotype and environment affect kernel hardness values, with genotype having a greater effect (Shihua 2003; Shihua et al. 2005). For example, puroindoline genes that were mapped to the Ha locus on the wheat chromosome 5D (Giroux & Morris 1998) explain 63.2% of the total variation in hardness (Sourdille et al. 1996).
Vromindolines are proteins coded for by the Vromindoline (Vin) gene family, which had been investigated in Avena species at different ploidy levels (except for Avena nuda) and shown to be responsible for the soft endosperm texture of oats (Gazza et al. 2015). In A. sativa, three Vin-1 genes, three Vin-2 genes, and two Vin-3 genes have been characterized and assigned to A, C, or D genomes based on similarity to their counterparts in diploid species (Gazza et al. 2015). The sequences of various vromindoline proteins of different species differ mainly due to amino acid changes caused by single base pair variations (Alfieri et al. 2014; Gazza et al. 2015).
In this study, A. nuda oats were planted in two ecological areas for three consecutive years (2019–2021) to obtain an adequate amount of mature seeds for hardness measurements. Materials with significantly different hardness values were selected for gene cloning, expression pattern analysis, allelic variation analysis and development of molecular markers related to grain hardness. This study characterized Vin-1 and Vin-3 genes in A. nuda, providing information for future research on the control of the grain texture of oat.
Materials and methods
Plant material
A total of 260 A. nuda, containing cultivars, landraces, and varieties imported from abroad, were planted in Bashang of Hebei Province (114°42′54″ E, 41°4′6″ N) and Ulanqab of Inner Mongolia (113°13′59″ E, 40°56′50″ N) in 2019, 2020, and 2021 (Supplementary file 1). The elementary plot of 1 m2 consisted of three rows, 30 cm apart, sown with 350 germinating kernels/m2.
Kernel hardness determination of oat
The grain harvested at two locations in 2019, 2020, and 2021 were tested for kernel hardness values. Broken, coarse, and small kernels were discarded, and one hundred grains were randomly selected for repeated tests of kernel hardness, which were evaluated by TA (TA-XT PLUS) (Jianghong 2022).
Preparation of paraffin sections and scanning electron microscopy (SEM) of mature grains
Mature grains were immersed in 4% paraformaldehyde fixative solution and breezed overnight at 4 ℃. The samples were sent to Beijing GenePool Biotechnology Co., Ltd. for paraffin sectioning and staining with hematoxylin. Mature, dry grains of naked oats were directly cut with a sharp blade. Sections of 1 mm were cut from each sample, and the bottom of the sample was fixed on the sample table with conductive adhesive. The samples were viewed using a TM4000pLUS scanning electron microscope (SEM) operating at 10 kV. The magnification level is 600x.
DNA extraction and PCR amplification
Genomic DNA was isolated from young leaves of all tested materials using the cetyltrimethylammonium bromide (CTAB) method (Turaki et al. 2018). The sequences of gene-specific primers are listed in Table 1. PCR reactions were performed in a Veriti™ 96-Well Thermal Cycler in a total volume of 25 μL. The reaction systems included 2.5 μL PCR buffer, 0.2 μM of each dNTPs, 10 pmol of each primer, 2.5 units of TransTaq Hifi DNA polymerase, and 1 μg of template DNA. The conditions for PCR amplification were 94 ℃ for 5 min, followed by 30 cycles of 94 ℃ for 1 min, 58–68 ℃ for 30 s, 72 ℃ for 30 min, and a final extension of 72 ℃ for 7 min. PCR products were analyzed on 2% agarose gels using GelDoc XR + with Image Lab Software.
Amplification products were purified from agarose gels with the EasyPure Quick Gel Extraction Kit. DNA amplification, cloning, and sequencing were performed at least three times for each genotype. Clones were sequenced by Huada Gene Co., Ltd (Shenzhen, China).
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted from natural mature seeds and seeds of A. nuda cvs HX367 at 3, 7, 14, 21, 28, 35 days post-anthesis (DPA). The root, stem, flag leaf, leaf sheath, and sterile lemma were also collected at the grain filling stage for RNA extraction. Plant samples were immediately frozen in liquid nitrogen and stored at − 80 °C. TransZol Plant kit was used to extract RNA from grains, and the TransZol Up Plus RNA Kit was used to extract RNA from other tissues. A Nanodrop 2000 was used to detect the concentration and quality of RNA. RNA integrity was assessed by comparing the relative intensities of the 28S and 18S rRNA bands in 1.2% (w/v) agarose gels. cDNA was synthesized from 1 μg of the total RNA using TransScript® One-step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech) according to the manufacturer’s instructions.
Semi-quantitative RT-PCR reactions were performed in a Veriti™ 96-Well thermal cycler in a total volume of 25 μL. The reaction conditions were 94 ℃ for 3 min, followed by 28 cycles of 94 ℃ for 30 min, 56–62 ℃ for 30 s, 72 ℃ for 20 s, and a final extension of 72 ℃ for 7 min. Values were normalized to the amplification rate of the actin gene as a constitutively expressed internal control (Tao et al. 2013). The RT-PCR products were separated on a 2.0% agarose gel. Three replicates were performed for each sample.
Sequence alignment and bioinformatics analysis
The sequence analysis of the Vin genes was performed in the genome database of OT3098 published on the GrainGenes website at https://wheat.pw.usda.gov/GG3/graingenes_downloads/oat-ot3098-pepsico. Protein secondary and tertiary structures were predicted using the online website at https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html and https://swissmodel.expasy.org/interactive. Vin sequences of diploid, tetraploid, hexaploid oats, wheat, barley, and triticale were downloaded from the NCBI Nucleotide database. MEGA 7 was used to construct the Neighbor-joining evolutionary tree with a bootstrap value at 1000 replicates.
Development of CAPS marker
DNAMAN software was used to compare and analyze the sequences of the sequencing results. The sequence files were submitted to Dna SP v.5.0 for extraction of variant sites, and haplotype analysis was performed on all variant sites. DNAMAN software was used to analyze whether there was a suitable restriction site near the SNP position of the sequence. Restriction endonucleases can recognize the difference of a single base, and the polymorphism of the restriction site can be used to establish the target. According to previous studies, specific primers of V1C6 gene were designed and synthesized (Table 2). PCR reactions were performed in a VeritiTM 96-Well Thermal Cycler in a total volume of 25 μL. The reaction systems included 2.5 μL PCR buffer, 0.2 μM of each dNTPs, 10 pmol of each primer, 2.5 units of TransTaq Hifi DNA polymerase, and 1 μg of template DNA. The conditions for PCR amplification were 94 ℃ for 5 min, followed by 30 cycles of 94 ℃ for 1 min, 62 ℃ for 30 s, 72 ℃ for 30 min, and a final extension of 72 ℃ for 7 min. Enzyme digestion reaction were performed in a FlyCut® EcoRI in a total volume of 20 μL. The reaction systems included 1 μg × PCR product, 2 μL 10 × FlyCut® Buffer 0.5 μL FlyCut® EcoRI and corresponding Nuclease-free Water. The conditions for enzyme digestion reaction was 37 ℃ for 15 min in metal bath. The digested products were detected by 2% agarose gel electrophoresis.
Subcellular localization
The AnVin-1C6 and AnVin-3C2 genes were fused with green and red fluorescent protein genes, respectively, to make the recombinant expression vectors pBWA(V)HS-V1C6-GLosgfp and pBWA(V)HS-V3C2-mKATE. The recombinant vectors were transformed into Agrobacterium GV3101 by electroporation. An empty vector was used as a control. Tobacco plants were injected at the lower epidermis of the leaves with a one mL syringe with the tip removed and marked. The injected tobacco plants were cultured in low light for two days. The marked tobacco leaves were mounted onto glass slides, observed under a laser confocal microscope, and photographed.
Statistical analysis
One-Way ANOVA in SPSS 21.0 software was used to conduct one-way analysis of variance on the experimental data and LSD method was used to conduct multiple comparisons to analyze the correlation between different haplotypes and oat kernel hardness.
Results
Cellular structure of grains with different hardness values in Avena nuda
The hardness values for HX317 (soft), huazao2hao (medium), and HX320 (hard) were determined by TA, and they were significantly different (Fig. 1a). Paraffin sections showed that the internal structure of A. nuda was composed of a seed coat, pericarp, aleurone layer, subaleurone layer, and starchy endosperm. The aleurone layer in each oat was composed of a single layer of cells of similar cell sizes. The structure difference of kernels with different hardness was manifested in the sub-aleurone layer and the inner floury endosperm. The sub-aleurone layer of soft oats is not obvious. The cell length was 47.8 μm ± 7.6, and even the secondary powder layer is missing in some parts. The sub-aleurone layer of hard oat had a distinct structure, with long cellsobvious structure and long cells (137.3 μm ± 7.3) inserted into the intercellular spaces of the endosperm. Endosperm cells of soft oat were arranged neatly and loosely, while those of hard oat were more closely arranged. The aleurone cells and endosperm cells of HX317 kernels were well-ordered. The arrangement of the aleurone cells became more disordered with increased kernel hardness value.
Kernel hardness and paraffin sections. a Measure of kernel hardness for HX317, huazao2hao, and HX320. Results are presented as mean and SD; n = 100. ** p < 0.01. b–d Paraffin section of mature grains in cross-section of HX317, huazao2hao and HX320. Aleurone layer and part of the inner endosperm of the kernel (red frame). Bar, 500 μm. The magnification levels of b–d are 3.3x. e Cell length of the subaleurone layer for HX317, huazao2hao, and HX320. Results are presented as mean and SD; n = 6. ** p < 0.01. f–h Magnified view of the aleurone layer and part of the inner endosperm of HX317, huazao2hao and HX320. The magnification levels of f–h are 10x. Bar, 100 μm
Ultrastructure of the endosperm of naked oats with different hardness.
SEM of the endosperm structures of HX317 and HX320 kernels was performed. The structures of the central endosperm and subaleurone layers of HX317 were loose with large gaps (Fig. 2a and b). Starch granules were primarily in the form of single and small grains (Fig. 2a). The structures of the central endosperm and subaleurone layers of HX320 were compact with small gaps (Fig. 2c and d). The starch granules were large and tightly bound to the surrounding protein matrix (Fig. 2c). The structure of the subaleurone layer of HX320 near the grain cortex was also more compact than that of HX317 (Fig. 2b and d).
Isolation and sequence analysis of AnVin-1 and AnVin-3 genes in Avena nuda L.
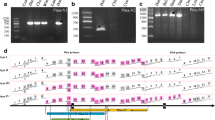
Genomic DNA and cDNA from HX367 were amplified using primer pairs Vin-1F/R and Vin-3F/R (Table 1). The sizes of the amplified AnVin-1 and AnVin-3 products at the gDNA and cDNA levels were the same (Fig. 3), indicating that these genes in A.nuda included a single complete ORF with no introns. Multiple sequence alignment analysis showed that the AnVin-1 and AnVin-3 genes of naked oats existed as multiple copies. The multiple sequences of AnVin-1 were named AnVin-1A1, AnVin-1A2, AnVin-1A3, AnVin-1C1, AnVin-1C2, AnVin-1C3, AnVin-1C4, AnVin-1C5, AnVin-1C6, AnVin-1C7, AnVin-1C8, AnVin-1C9, AnVin-1C10, AnVin-1D1, AnVin-1D2, AnVin-1D3, AnVin-1D4, and AnVin-1D5. The multiple sequences of AnVin-3 were named AnVin-3C1, AnVin-3C2, AnVin-3C3, AnVin-3C4, AnVin-3C5, AnVin-3C6, AnVin-3C7, AnVin-3C8, and AnVin-3C9.
Multiple sequence alignment analysis showed that the homology among the AnVin-1 genes ranged from 96.13 to 99.77%, and the homology of AnVin-3 genes ranged from 94.17 to 99.30%. The multi-copy sequences showed single base pair differences. There were 3 SNPs in AnVin-1A, resulting in 2 missense mutations and 1 synonymous mutation (Supplementary file 2, Table 2). There were 8 SNPs in AnVin-1D, resulting in 5 missense mutations and 3 synonymous mutations (Supplementary file 3, Table 2). There were 11 SNPs in AnVin-1C, resulting in 4 synonymous mutations, 4 missense mutations, and 5 nonsense mutations. One of the missense mutations in AnVin-1C (K/Q) occurred in the tryptophan-rich region of “WPWKWWK” (Fig. 4, Table 2). There were 32 SNPs in AnVin-3C, resulting in 15 synonymous mutations, 17 missense mutations, and one nonsense mutation. One of the missense mutations in AnVin-3C (K/R) also occurred in the “WPWKWWK” region (Fig. 5, Table 3).
The amino acid sequence similarity of between VinA1a in A.sativa and AnVin-1A1 in A.nuda was 100%. VinA1a differed from AnVin-1A2 and AnVin-1A3 by one amino acid each (Table 2). Compared with VinC1a in A.sativa, AnVin-1C had 5 amino acids that were unique to A. nuda. Similarly, AnVin-1D had 3 unique amino acids (Table 2). VinC3a in A.sativa has 2 isoforms containing 6 amino acid differences (Table 3). The 9 sequences of AnVin-3C in A.nuda contain this 6 amino acid, while the 27th amino acid in the coding region is a specific amino acid for A.nuda (Table 3).
Genomic marker development of AnVin-1 gene in Avena nuda L.
The upstream and downstream 500 bp sequences of AnVin-1A, AnVin-1C, and AnVin-1D coding sequences were obtained from the predicted sequence of AnVin-1 in the OT3098 database. (Supplementary file 4). Primers were designed based on the specific site of AnVin-1 between three genomes. PCR amplification was performed on HX367 gDNA using the primers Vin-1A1F/R, Vin-1C1F/R, and Vin-1D1F/R (Table 1, Fig. 6).
Bioinformatics analysis of AnVIN-1 and AnVIN-3 proteins of Avena nuda L.
AnVIN-1 and AnVIN-3 were found to be 147 and 142 amino acids long, respectively. Both proteins included a “WPWKWWK” tryptophan-rich region and 10 cysteines. Conserved domain searching revealed that AnVIN-1 and AnVIN-3 belonged to the seed storage protein (SS) subfamily of the alpha-amylase inhibitors (AAIs) superfamily. The predicted secondary structures of AnVIN-1 and AnVIN-3 contained α helices, β sheets, random coils, and extended strands (Fig. 7a). The predicted tertiary structures of AnVIN-1 based on the A, C, and D genomes were similar, but there were differences in the tertiary structure of AnVIN-1 and AnVIN-3 proteins (Fig. 7b).
A phylogenetic tree was constructed with Vin-like gene sequences in diploid, tetraploid, hexaploid of oat, and wheat, barley, and triticale. The analysis showed that multiple distinct copies of each of the AnVin-1A, AnVin-1C, AnVin-1D, and AnVin-3C gene sequences clustered together. AnVin-1A, AnVin-1C, and AnVin-3C were clustered with diploid A and C genomes, respectively. AnVin-1A and AnVin-1D genes were clustered together in the same branch, indicating that they were more closely related. AnVin-1 and AnVin-3 genes were on different branches, indicating that they represented two different AnVin genes (Fig. 8). The AnVin genes were more closely related to their counterparts in other species of the oat genus than the more distantly related wheat, barley, and triticale (Fig. 8). In addition, the Ain gene in A. sativa was more closely related to the Pin, Hin and Sin genes in wheat, barley, and triticale than to the AnVin gene in A. nuda (Fig. 8).
Phylogenetic analysis of AnVin-1, AnVin-3 Vin-like genes in Avena and related species. The green circles represent the AnVin-1A genes; the yellow circles represent the AnVin-1D genes; the blue triangles represent the AnVin-1C genes; and the red diamonds represent AnVin-3C. The purple squares and red line diamondsrepresent the Vin and Ain gene in A. sativa. Black squares represent the Pin genes in wheat; black triangles represent the Hin genes in barley; and black diamonds represent the Sin genes in triticale
Expression pattern of AnVin-1 and AnVin-3 in Avena nuda L.
Semi-quantitative RT-PCR analyses among different tissues of oat showed that AnVin-1C, AnVin-1D, and AnVin-3C were expressed in all tested tissues (Fig. 9). However, AnVin-1A was not expressed in stems and mature kernels (Fig. 9a). The expression of AnVin-3C was the highest in leaf sheaths (Fig. 9a). The expression levels of AnVin-1 and AnVin-3 first increased and then decreased at different stages of grain development, with the highest expression levels at 14 DAP (Fig. 9b). From the 260 oats, 5 extremely soft and 5 extremely hard materials were selected for hardness measurements and differential gene expression analysis. The results showed that there was little difference in the expression levels of AnVin-1A and AnVin-1D in kernels at 14 DAP among the 10 materials. However, the expression levels of AnVin-1C and AnVin-3C in soft oat were higher than those in hard oat (Fig. 9c).
The expression pattern of AnVin-1 and AnVin-3. a Expression in different tissues. Lanes 1–6 represent roots, stems, flag leaves, leaf sheaths, protective glumes, and mature grains, respectively. b Expression at different developmental stages. Lanes 1–7 represent 3, 7, 14, 21, 28, and 35 DAP and mature grains after flowering, respectively. c Expression in 14 DAP kernels of different hardness values, lanes 1–10 represent HX317, HX88, HX106, HX54, HX344, HX310, HX268, HX21, HX265, and HX320, respectively
Gene markers development for different copy sequences of AnVin-1 and AnVin-3
Due to the high sequence similarity of the three AnVin-1A sequences, no specific primer could be designed for single-copy sequences. The primers were designed based on specific sites in the 10 AnVin-1C sequences. The primers Vin1C-133F/R, Vin1C-3F/R, Vin1C-94F/R, and Vin1C-101F/R were used to obtain the sequences of AnVin-1C2, AnVin-1C6, AnVin-1C8, and AnVin-1C10, respectively (Fig. 10a). The primers Vin1D-9F/R, Vin1D-17F/R, Vin1D-56F/R, and Vin1D-29F/R were used to obtain the sequences of AnVin-1D1, AnVin-1D2, AnVin-1D3, and AnVin-1D5, respectively (Fig. 11b). The primers Vin3C-16F/R, Vin3C-26F/R, Vin3C-4F/R, Vin3C-12F/R, Vin3C-59F/R, Vin3C-41F/R, and Vin3C-49F/R were used to obtain the sequences of AnVin-3C1, AnVin-3C2, AnVin-3C3, AnVin-3C4, AnVin-3C5, AnVin-3C7, and AnVin-3C8, respectively (Fig. 11c). These primers were the gene markers of the sequences of AnVin-1 and AnVin-3.
Identification of single copy sequence of AnVin-1 and AnVin-3. a Different copy sequence identification of AnVin-1C. Lanes 1–10 represent AnVin-1C1 to AnVin-1C10 sequences. b Different copy sequence identification of AnVin-1D. Lanes 1–5 represent AnVin-1D1 to AnVin-1D5 sequences. c Different copy sequence identification of AnVin-3C. Lanes 1–9 represent AnVin-3C1 to AnVin-3C9 sequences
Haplotype analysis of AnVin-1 and AnVin-3 genes in naked oats
Specific primers were used for the identification of copy sequences in the 5 extremely soft and 5 extremely hard materials (Table 1, Fig. 10). Only AnVin-1C6, AnVin-1C8, AnVin-1D2, AnVin-1D3, AnVin-3C2, and AnVin-3C4 could be identified in the 10 materials. The other sequences appeared in only individual materials. Among the sequences, only AnVin-1C6 and AnVin-3C2 had three haplotypes in the 10 materials, which had polymorphisms at 153 bp (T/C), 598 bp (G/A), and 648 bp (C/T), respectively, and these changes were linked. The polymorphism at 153 bp was located in the coding region but did not cause a change in the amino acid sequence. The other two polymorphisms were located in the 3’-UTR region (Fig. 11, Supplementary file 5). There were three haplotypes of AnVin-3C2 in the 10 materials, with linked changes at 207 and 291 bp (Fig. 12, Supplementary file 6). The SNP at 207 bp was a synonymous mutation, while the SNP at 291 bp caused a missense mutation (Cys to Arg).
Development and validation of molecular markers for grain hardness in Avena nuda L.
An EcoRI cleavage site was found at site 598 bp of AnVin-1C6. Amplified samples from the 10 materials were digested with EcoR I. The haplotype resistant to EcoRI digestion was named AnVin-1C6b, while the haplotype that could be digested into two fragments was named AnVin-1C6a (Fig. 13). The amplified fragments of 56 softest and 56 hardest naked oats were digested with EcoR I (Table 4). Among the 112 tested naked oats, 79.5% (89/112) were of the AnVin-1C6a genotype, and 20.5% (23/112) were of the AnVin-1C6b genotype. The average hardness values of the 56 soft and hard oats were 26.69 and 28.23 N, respectively. Correlation analysis and one-way ANOVA showed that the hardness values of the two genotypes had a low correlation (R = 0.120), and the difference was not significant (P = 0.207). The sum of squared deviations between groups accounted for 1.44% of the total sum of squared deviations (Table 5). The results showed that the CAPS marker had a very low interpretation of the hardness variation in these 112 natural populations.
Subcellular localization of AnVin-1C6 and AnVin-3C2 genes
AnVin-1C6 and AnVin-3C2 were fused to the N-termini of green and red fluorescent protein genes, respectively, to construct fusion expression vectors (Supplementary file 7). These vectors were transformed into tobacco leaf cells. The green fluorescence of AnVin-1C6 and the red fluorescence of AnVin-3C2 may be concentrated in cytomembrane (Fig. 14). The expression effect of both genes is weak.
Conclusion
In the present study, we found that the structural differences of A. nuda with different grain hardness were primarily reflected in the sub-aleurone layer and the internal endosperm. Multiple copies of AnVin-1 and AnVin-3 genes exist in A. nuda, and their expression is not seed-specific. The expression levels of the two genes were highest among the 14 DAP seeds. The expression levels of AnVin-1C and AnVin-3C were higher in soft oat than in hard oat. CAPS marker developed from the two genotypes of the AnVin-1C6 sequence from the 10 materials could explain 1.44% of the hardness variation.
Discussion
Vin genes are conserved within the genus Avena, but research on related genes in A. nuda is sparse. In this paper, we focused on the characterization of Vin-related genes in A. nuda. Both AnVin-1 and AnVin-3 had a tryptophan-rich region of “WPWKWWK,” which is consistent with the structure of indoline proteins. This region is related to its polar lipid affinity to starch (Schibli et al. 2002). Phylogenetic tree analysis found that the Ain gene was more closely related to the Pin gene in wheat than to the Vin gene in oat, consistent with the results of Gazza (Gazza et al. 2015) It was demonstrated that the role of Ain gene in oat grain hardness was insignificant through Western blot and that Vin had a greater effect on grain hardness than Ain (Gazza et al. 2015). The expression differences of AnVin in different hardness materials primarily occurred in the C genome, which may be related to its phylogenetic relationships.
The structure of oat kernels consists of seed coat, pericarp, aleurone layer, subaleurone layer and starchy endosperm. From the perspective of cell structure, the cell size of the aleurone layer is similar, which is consistent with Zhang Ruiqi’s research (Ruiqi et al. 2011) on the relationship between the hardness of wheat grains and their microstructure,which found that there is no significant difference in the aleurone layer of wheat grains with different hardness. The morphology and arrangement of the subaleurone layer will have a significant impact on the grain hardness, and the arrangement of the endosperm cell structure will affect the grain firmness. From the perspective of microstructure, the aleurone layer cells of soft oats are arranged neatly, while the cells of hard oats are more disorderly. The endosperm structure of hard oat was tight, and the starch granule was full. The protein and starch granule, starch granule and starch granule squeezed each other. The void was small, and the starch granule showed irregular shape. The starch granules in soft oats are loosely arranged, and there are large gaps between protein and starch granules, and between starch granules. The tightness of protein and starch granules is related to hardness, which is consistent with the research conclusion of wheat hardness (Ma et al. 2016).
CAPS Marker is a molecular marker technology that combines PCR and enzyme digestion technology, and a relatively simple method to detect SNPs site variation. Restriction endonucleases can recognize the difference of a single base, and the polymorphism of the restriction site can be used to establish the target. In this study, although there are multiple SNP sites, some SNPs do not cause changes in the recognition sites of restriction endonucleases, so that multiple sites cannot be directly detected by endonucleases. The developed CAPS marker only explained 1.44% of the phenotypic variation rate, which may be related to the gene conservation and population size. The verification of molecular markers of grain hardness needs to be further carried out in larger natural populations or recombinant inbred lines.
We found that the AnVin gene had high sequence similarity in materials with different hardness values. Polymorphisms caused difficulty in developing specific primers. Due to the high homology of each sequence, ARMS-PCR method was used to design primers (Yijia et al. 2013). The AnVin-1 was not seed-specific, indicating AnVin-1 might also function in other tissues. Puroindoline (PIN) was shown to inhibit growth and kill a variety of bacteria and fungi (Morris 2019). PINA can rupture mycelium cell membranes, damage mitochondria, and fragment or translocate DNA of Aspergillus flavu, thereby affecting the storage characteristics of wheat (Ang 2020). The antibacterial effect of PINA is greater than that of PINB. PIN exerts its antibacterial effect primarily by lysing the pathogenic bacterial plasma membrane with specific lipid components (Yingjie 2013). VINs may also have functions similar to PINs and located outside the cell membrane. In this study, we found that the AnVin-1 and AnVin-3 proteins may be were located in cytomembrane. The results indicated that the AnVIN-1 might be a nucleoprotein and AnVIN-3 might be a secreted protein. AnVIN-1 and AnVIN-3 may work in different ways in the mechanism of grain hardness formation in naked oat.
We present a preliminary investigation of AnVin genes in A. nuda, using Vin genes as comparator references, which will provide information for future research on the grain texture of A. nuda.
References
Alfieri M, Gazza L, Pogna NE, Redaelli R (2014) Gene sequences of vromindolines in Avena species. Genet Resour Crop Evol 61(8):1481–1490. https://doi.org/10.1007/s10722-014-0123-4
Ang L (2020) Expression of wheat PINA protein in vitro and its antifungal effect against Aspergillus Flavus. Dissertation, Henan University of Technology
Chunqing Z (1992) Study on relation between texture and chemical composition of wheat. Food Storage 21(1):33–38
Gazza L, Taddei F, Conti S, Gazzelloni G, Muccilli V, Janni M (2015) Biochemical and molecular characterization of Avena indolines and their role in kernel texture. Mol Genet Genom 290(1):39–54
Giroux MJ, Morris CF (1998) Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b. Proc Natl Acad Sci USA 95(11):6262–6266
Greenwell P, Schofield JD (1986) A Starch granule protein associated with endosperm softness in wheat. Cereal Chem 63:379–380
Jianghong A (2022) Grain hardness and allelic variation and hardness related molecular marker development of Vin-1/3 genes in Avena nuda[D]. Inner Mongolia Agricultural University
Jianghong A, Wenjing Z, Yinglin Z, Bing H, Jinsheng N (2020) Research progress on grain hardness of wheat crops. J North Agric 48(4):40–47
Jianghong A, Wenjing Z, Xiaohong Y, Jinsheng N, Yan Y, Mingxia Y, Bing H (2021) Comparison of two methods for kernel hardness determination of naked oats. Crops (06): 28–35
Ma D, Qin H, Ding H, Jian Z, Guo T (2016) Surface lipids play a role in the interaction of puroindolines with wheat starch and kernel hardness. Cereal Chem 93(5):523–528
Morris CF (2019) The antimicrobial properties of the puroindolines, a review. World J Microbiol Biotechnol Bull 35(6):86. https://doi.org/10.1007/s11274-019-2655-4
Ruiqi Z, Man R, Shouzhong Z, Lin H, Weigang X (2011) Relationship between the wheat grain hardness and the endosperm composition and microstructure in a RIL population. Sci Agric Sin 9:1753–1762
Schibli DJ, Epand RF, Vogel HJ, Epand RM (2002) Tryptophan-rich antimicrobial peptides: comparative properties and membrane interactions. Biochem Cell Biol 5:80
Shihua G (2003) Biochemical and molecular markers of grain hardness in chinese common wheat (Triticum aestivum L.). Dissertation, Shandong Agricultural University
Shihua G, Zhonghu H, Qing M, Honggang W (2005) Wheat grain hardness review. J Triticeae Crops 25(2):107–111
Sourdille P, Perretant MR, Charmet G, Leroy P, Bernard M (1996) Linkage between RFLP markers and genes affecting kernel hardness in wheat. Theor Appl Genet 93(4):580–586
Tao Y, Shanshan S, Shiqing G, Yimiao T, Changping Z (2013) Cloning of AsRBP1 gene from oat and analysis on its expressions to stresses. J Triticeae Crops 33(02):217–223
Turaki AA, Ahmad B, Magaji UF, Abdulrazak UK, Yusuf BA, Hamza AB (2018) optimised cetyltrimethylammonium bromide (ctab) dna extraction method of plant leaf with high polysaccharide and polyphenolic compounds for downstream reliable molecular analyses. Afr J Biotechnol 16(24):1354–1365
Yijia Z, Yanying Q, Yuanyuan X, Quanjia C, Wenlin F, Shunyao M, Weiwei L (2013) A Study on cotton SNP typing by ARMS-PCR. Xinjiang Agric Sci 50(12):2182–2188
Yingjie M. (2013) Construction, expression and antibacterial activity analyses of wheat puroindoline a artificial mutants and its functional analyses in transgenic wheat. Dissertation, Huazhong University of Science and Technology
Acknowledgements
Thanks to the Institute of Crop Science, Chinese Academy of Agricultural Sciences for providing a portion of the oat materials.
Funding
We appreciate the funding support from National Key Research and Development Project of China (2022YFE0119800), Funding form Key Laboratory of Germplasm Innovation and Utilization of Triticeae Crops at Universities of Inner Mongolia, and team funding of the School of Life Sciences of Inner Mongolia Agricultural University (TD202103).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JA, JN, MC, HL, YY, and BH. The first draft of the manuscript was written by JA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, Jh., Nan, Js., Liu, Hy. et al. The expression characteristics and allelic variation of AnVromindoline-1/3 in Avena nuda L.. Euphytica 219, 127 (2023). https://doi.org/10.1007/s10681-023-03251-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-023-03251-9